
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:stions bartleby
Fort Lewis College - CHEM 150 x
+
php?id-8911716
HW 6: Molecular Gec
Dates HW 6: Molecular Geometries
Lx Give Up?
Resources
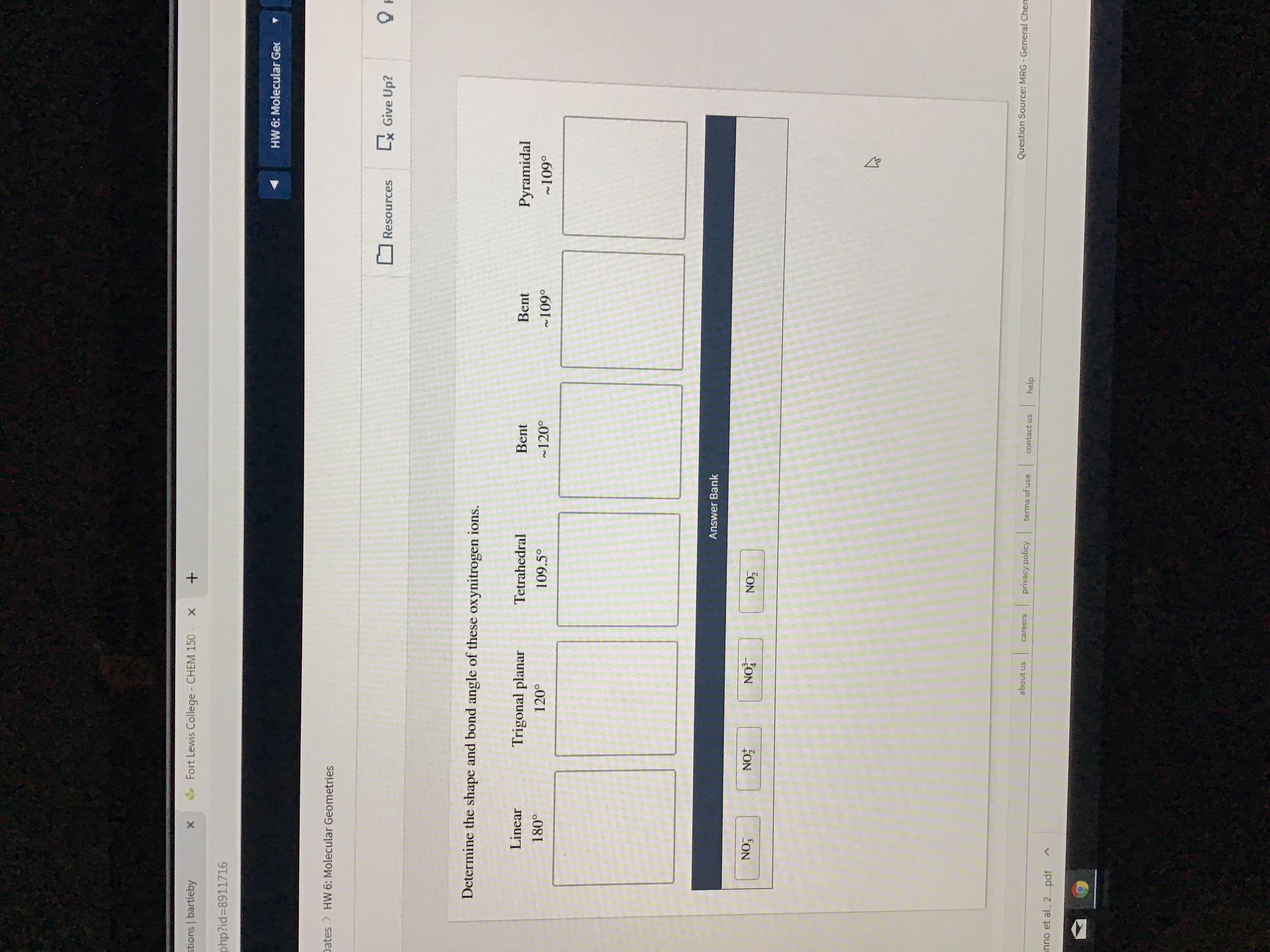
Determine the shape and bond angle of these oxynitrogen ions.
Linear
Trigonal planar
Tetrahedral
Bent
Bent
Pyramidal
180°
1200
109.5°
120°
109°
~109°
Answer Bank
NO3
NO
NOT
NO7
Question Source: MRG -General Chem
about us
|
privacy policy
careers
terms of use
contact us
help
nno et al., 2....pdf
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- Part A Predict whether each of the following molecules is polar or nonpolar. Drag the appropriate items to their respective bins. Polar Submit SeF4 NH3 Request Answer GaH3 CH,I XeF 11 Nonpolar CBTA Reset Help Pearsonarrow_forwardс C O Macmillan L $ 4 Classify each bond as ionic, polar covalent, or nonpolar covalent. D R F P-F S-F V % 5 Ionic 21 T C-H G Q G Search or type URL ^ 6 Fe-0 B MacBook Pro Y stv H S-S & 7 U J Polar covalent * 00 Answer Bank 8 Na-N N M + < I ( 9 K O-Bri 0 < I ) - O L Nonpolar covalent Cs-Cl P A .... I-I { لالہ + 11 S 11arrow_forwardPolar or Electron Pair Shape Molecular Shape Approximate Bond Angles Dot Structure Bonding Sketch Molecule Non-polar CIF3 3- POA IF5 clo, Cs2 XeF 4 C2H;Br Experiment 12 Report Sheet (continued) 121arrow_forward
- Chrome ← → C canvas K File Edit Aktiv Chemistry mcat prep tab app.101edu.co View History Bookmarks caps lock P pearson esc HM lab Draw a resonance structure that places a pi bond in a different position. Include all lone pairs in your structure. Incorrect, 3 attempts remaining :O: :0: Select to Draw ! 1 k Q A Profiles Tab Window Topic: Module 1 Discussion - d x chemistry Q 2 Q 45 X Login W S #3 Help To Do Assignment... C G 1-butanol boiling point - Googl X + E Problem 42 of 56 Incorrect, 3 attempts remaining You have incorrectly identified the appropropriate number of lone-pairs within this compound. Review the bonding pattern of each atom, taking into consideration the number of lone- pairs and formal charges. MAY 14 pinter at NEU D $ 4 Please select a drawing or reagent from the question area Retry R do 50 APA Citation Gene... % m G Search or type URL F Ը MacBook Pro T 6 2 G Paraphrasing Tool... Y ∞ étv 7 I اله # CO U MCATBRC Jarrow_forwardTable 3 Molecules with Multiple Central Atoms C₂H4 H₂O2 1 1 Molecule Number of Bond Pairs Number of Lone Pairs Number of Electron Domains Molecular Geometry (Shape) Bond Angle Polar or Non- Polar 1 / 1 1 Data Results / Analysis: 1 / 1 CH3OH 1 1 1 1 1 1. What determines which atoms are the central atoms? CH3NH₂ 1 / 1 1 1arrow_forwardA) 32 B) 30 :9: C) 34 D) 33 E) 31 F2 Determine the number of valence electrons in PO43 and then draw the corresponding Lewis structure (by following the octet rule on all atoms). 80 F3 F4 ܀ F5 MacBook Air F6 9 F7 * F8 F9 + Click to draw a new structure F10 ( F11 + F12arrow_forward
- Can you please show step by step how to do this on Lewis structuress on a piece of paper.arrow_forwardplease answer neatly and clear and answer super super fast i really need it beofre the end of today its really important and urgent please try to answer everything please try to draw everything can you please please draw the sigma bond for H2O2 draw the sigma bond and pi bond for HCN draw the sigma bond and pi bond for N2Oarrow_forwardI need help with my homework questionsarrow_forward
- neucommon-assessment-delivery/start/49930810487action%3Donresume&submissionld3D575321765 POSSIBLE P Based on the electronegativity values, determine which type of bond is formed between the following elements. Increasing electronegativity electronegativity <1.0 1.0 s electronegativity < 2.0 2.0 s electronegativity < 3.0 Не 2.20 10 Ne 08. 13 Li 6. B C Be 0.98 1.57 12, Mg 131 21 2.04 13 Al 1.61 31 Ga 1.81 2.55 3.04 344 3.98 17 CI 2.58 3.16 35 Br 3.0 s electronegativity < 4.0 14 Si 1.90 32 Ge 2.01 50 Sn 1.96 2.05 82 TI Pb 1.8 15 16 18 Ar 11 Na 0.93 19 2.19 33 34 Se 36 Kr 30 Zn 1.65 48 Cd 1.69 80 Hg 1.9 29 Cu 28 Ni 1.91 46 Pd 2.28 2.20 78 Pt 2.2 110 27 26 Fe 1.83 44 25 Mn 22 23 24 20 Ca 1.00 As 2.18 Ti 1.54 40 Zr 1.33 72 Hf 1.3 104 Rf Co Cr 1.55 43 1.66 1.88 1.90 2.55 2.96 0.82 37 Rb 1.36 39 1.63 41 Nb 54 Xe 51 52 53 49 In 1.78 47 45 Ru 22 76 Os 2.2 108 Hs 42 38 Sr Sb Te Tc 2.10 75 Rh Ag 1.93 Mo 2.1 2.66 2.16 0.95 1.22 57 La 1.1 89 Ac 1.1 1.6 73 Ta 15 105 Db 0.82 86 Rn 77 79 81 83 84 85 55…arrow_forward~ Draw the Lewis structure for the ozone esc Explanation ! A 1 N 2 Check W # S #3 X 28 option command (03) molecule. Be sure to include all resonance structures that satisfy the octet rule. E D $ 4 C R F % 5 T V ^ 6 MacBook Pro Y Ċ 3 B X & 7 G H Ċ Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privas U 5 N 8 00 J 1 ( 9 K M ) O O V B 28 Parrow_forwardH-Ñ-²0² -I Draw the Lewis structure of NH₂OH and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure. Click to edit molecule Question 3 of 24 H A) bent (120°) / trigonal pyramidal B) trigonal / bent (120°) C) trigonal pyramidal / bent (109.5°) D) linear / tetrahedral E) linear / trigonal planar Submit +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY