
Concept explainers
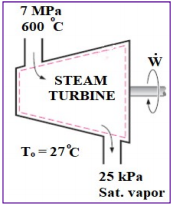
Steam expands in a steam turbine steadily at a rate of 32,000 kg/h, that enters the turbine
at 7 MPa and 600 oC (look to the Fig. 1); whereas, it leaves the turbine at 25 kPa as saturated vapor. If
this turbine generates a power of 6.5 MW at the ambient conditions of T0=27 oC, then,
a) Write the mass balance of the fluid entering and leaving this turbine.
b) Obtain the energy balance to determine the heat loss of the steam turbine,
c) Find the entropy generation using the entropy balance and determine the destroyed exergy using the
generated entropy,
d) Find the exergy destruction using the exergy balance ,
e) Find the maximum possible power output,
f) Determine the second-law efficiency (ηII) of the steam turbine,
Step by stepSolved in 2 steps with 2 images

- Steam enters a continuous flow turbine at a pressure of 8 MPa and a temperature of 450 ℃ and reaches a pressure of 70 kPa it is expanding. The mass flow rate of the steam is 200000 kg/hour and it is in the form of saturated steam at the outlet. By accepting the ambient temperature, 25℃ and pressure 100 kPa; a) The power potential of the steam at the inlet conditions b) Can you find the turbine power output for the situation where there are no irreversibilities? (Kinetic and potential energy ignore the changes).arrow_forwardRefrigerant R-134a at 200 kPa and 50 ⁰C enters an adiabatic diffuser steadily with a velocityof 140 m/s and leaves at 500 kPa and 150 ⁰C. Determine: a) The exit velocityb) The inlet to exit area ratioc) The inlet exit density ratio clear writing pleasearrow_forward200Kg/min superheated steam at 40 bar and 350C enters a turbine through a 7.5cm ID pipe. It exits at 5bar as saturated steam through a 5cm ID pipe neglect the change in potential energy of the system. What is the temperature of the outlet saturated system? How much energy is transferred to or from the turbine?arrow_forward
- 4) A boiler generates 600 kg of steam per hour. The enthalpy of feed water s 168 kj/kg and the enthalpy of steam is 2772 kj/kg. Neglecting PE KE cal the rate at which the heat is transferred.arrow_forwardI need help with a review porblem I cannot solve! thank you Steam enters an adiabatic turbine at 4 MPa, 800K and exits at 8 kPa, 90% quality. Assuming steady state, steady flow conditions, determine: a) The power output of the turbine (MW) if the mass flow rate is 63kg/s. b)The volumetric flow rate at the inlet (m3/s).arrow_forwardSteam to a turbine at a mass flow rate of 1.4 kg/s, 700 kPa pressure and 400 °C enters the temperature. Steam at 100 kPa pressure and 1.4 m3/kg specific volume exits the turbine. Heat transfer from turbine to environment 50 kW, with turbine Since the boundary temperature between the environment is 70 °C, a) Find the power produced by the turbine, entropy produced in the turbine and isentropic efficiency of the turbine. Note: The changes in kinetic and potential energies will be neglected and T (K) = 273 + °C will be taken.arrow_forward
- An internally reversible process occurs in a system during which Q = – 12 KJ, AU = – 79 KJ and Ah = - 111 KJ A. Find the work if the system is non- flow. B. Determine the work and the change of flow energy if the system is steady state, steady flow system with AEK 4 KJ. %3Darrow_forwardA steady stream of steam passes through an adiabatic turbine. It enters at 10 MPa, 450 °C and 80 m/s and at the exit it is 500 KPa, 40% quality and 30 m/s. The mass flow of the steam is 12 kg/s. a) With the power that comes out of the system, 252 100 W bulbs can be lit. Calculate the efficiency of the system. b) What is the area of the duct at the entrance? c) What is the volumetric flow at the outlet? d) What is the temperature of the steam at the outlet?arrow_forwardA certain ideal gas has a constant R = 38.9 ft-lff/lbm-R with k = 1.4. a) If 3 lbm of this gas undergoes a reversible nonflow constant volume process from 120 psia,740 oF to 140 oF find P2, ∆U, ∆H, Q, Work. b) If the process in “a” had been steady flow find work and ∆S.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





