
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please Answer all my Question .. Please help me , I don't want plagiarism.
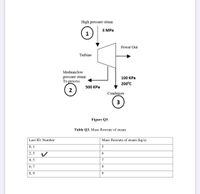
Transcribed Image Text:High pressure steam
З МРа
1
Power Out
Turbine
Medium/low
pressure steam
To process
100 КРа
200°C
500 КРа
Condenser
3
Figure Q3.
Table Q3. Mass flowrate of steam
Last ID. Number
Mass flowrate of steam (kg/s)
0, 1
2, 3
6.
4, 5
7
6, 7
8
8, 9
9

Transcribed Image Text:Q3.
Steam enters an industrial isentropic steam turbine at 3 MPa, and 7 percent steam at 500 kPa is
redirected to a heat exchanger to preheat the feed water with the turbine's second outlet. The
leftover steam travels to the condenser at a pressure of 100 kPa and a temperature of 200°C (Figure
Q3).
Consider the mass flow rate of steam in Table Q3 in relation to your last ID. Number in your
calculations.
(а)
Calculate the turbine's power output in kW using the necessary assumptions.
(b)
Describe the conditions under which a turbine can operate in an isentropic state. Compare
the power output of a real turbine with an isentropic turbine intuitively.
(c)
Due to irreversibility, the entropy of a real turbine process grows. To keep the entropy of
the steam at a low value when it leaves the turbine, it is recommended that some heat be
extracted from the steam via cold water circulation before it leaves the turbine. This will
keep the entropy at a low value when the steam leaves the turbine, and thus the work output
will increase. How do you rate this suggestion for boosting the turbine's efficiency?
(d)
Discuss the key processes of a steam turbine and the system's mechanical components.
Also, why isn't the steam from the turbine directly sent into the boiler and save the thermal
energy that is disepated in the condensor?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Calculate the number of finished goods (output) or N for the manufacturing process illustrated in the next page.arrow_forwardI asked for problems 6 and 7 to be answered, but I did not get a properly structured answered as the example shows on problem number 1. Here is the link to the questions I already had answered, could you please rewrite the answer so its properly answered as the example shows (Problem 1)? https://www.bartleby.com/questions-and-answers/it-vivch-print-reading-for-industry-228-class-date-name-review-activity-112-for-each-local-note-or-c/cadc3f7b-2c2f-4471-842b-5a84bf505857arrow_forwardExplain the term degree of indeterminacy? Give an example?arrow_forward
- 1. Why does a surface composed of a particular material get dirtied? 2. Why do engineering metals and alloys break, despite the fact that they can't be prevented from doing so? Please explain in full detail! Thank youarrow_forwardPlease answer in the column by using the answer provided.arrow_forwardIf the wrought stock material costs $60 per kg, what is the lost value due to the material removed, assuming scrap is sold for $3 per kg? Assume the density of titanium is 4.5 g/cm3. Briefly explain how you got your answer. Neglect costs of lubricant/coolant, chip handling, etc. Round your answer down to the nearest dollar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY