
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
No plagiarism Please!
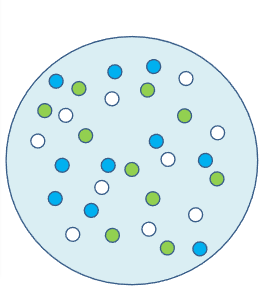
Station #6 = HClO4 (aq)
- For this acid, what does the white bead represent?________ blue bead? __________ 3rd Color Bead? _________
- Record your observations in the particulate drawing.
- How many acid molecules have broken into ions?
- Determine the % ionization for the acid.
- Write the ionization reaction for this acid. ________ → ______ + _______
Transcribed Image Text:O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the reaction of aqueous sodium nitrate and aqueous sulfuric acid, which of the following best describes the net ionic equation and driving force of the reaction? This reaction is not possibe because very high temperature is required to make the reaction feasible. O not enough information O Net ionic equation: 2Na+ (ag) + SO4 (ag) ----> 2- Na2SO4(s) Driving force: formation of a solid (Na2SO4) O Net ionic equation: no net ionic equation Driving force: formation of a gas (NO2) O Net ionic equation: no net ionic equation Driving force: formation of a strong electrolyte which then dissociates (HNO3)arrow_forwardPlease help me with the right answers. Please ! Carefully read the question before submitting just any answers , thanks.arrow_forwardSend help pls.arrow_forward
- Balance the following reaction. Enter a number in each blank. You must answer numerically, eg "1" not "one". Al+ HCI AICI3 + H2arrow_forwardTwo students are studying the Arrhenius reaction below: NAOH + Hz S04 acid → Na SOy + HzO base salt water Did the students identify the acid, base and salt correctly? If not, provide the correction.arrow_forwardPlease calculate the concentration of Cl in a solution of HCI with pOH = 4.49 Please express your answer to the second decimal place in scientific notation. (Note: be sure to show your work on your paper). Canvas doesn't understand x in scientific notation so 1.00x1012 should be input as 1.00 * 101 -12arrow_forward
- Sodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forwardexolain how to solve trial one so i can do the remaining trials on my own. thanksarrow_forward4-7arrow_forward
- SIMPLE REACTIONS Shamma V Determining the volume of base neede... A chem student weighs out 0.0999 g of acetic acid (HCH,CO,) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1100 M NAOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits. ||mL O ml. x10 dlo 18 Ar Explanation Check O 2021 Mcolaw- LUutatioI. All RightS RESEIVEU. Privacy Accessibilityarrow_forwardurces UIW Web Library Online Tips UIW Course Policies, Guidelines and Accommodations try 02 Fa 20 CHEM1310 02_Fa_20 Module 11 11. Reactions of Acids and Bases ... Identify types of reactions involving acids and bases Question Complete and balance the chemical equation for the neutralization reaction of phosphoric acid (H, PO,(aq)) and sodium hydroxide (NAOH(aq)). 4. • Write the chemical equation in molecular form (do not show dissociated ions). • Include the state (phase) of each chemical species. Provide your answer below: H,PO,(aq) +NaOH(aq) →D FEEDBACK MORE INSTRUCTION SUBMIT Content attribution >arrow_forwardSuppose a 0.031M aqueous solution of sulfuric acid (H₂SO4) is prepared. Calculate the equilibrium molarity of SO4. You'll find information on the properties of sulfuric acid in the ALEKS Data resource. Round your answer to 2 significant digits. IM S P Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY