
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
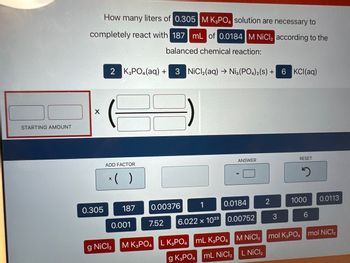
Transcribed Image Text:STARTING AMOUNT
How many liters of 0.305 M K3PO4 solution are necessary to
completely react with 187 mL of 0.0184 M NiCl₂ according to the
balanced chemical reaction:
X
0.305
2 K3PO4 (aq) +
ADD FACTOR
x( )
187
0.001
g NiCl₂
3 NiCl₂(aq) → Ni3(PO4)2(S) +
7.52
0.00376
0.0184
6.022 x 1023 0.00752
ANSWER
M K3PO4 L K3PO4 mL K3PO4
g K3PO4 mL NiCl₂
2
3
6 KCl(aq)
RESET
2
1000 0.0113
6
M NiCl₂ mol K3PO4 mol NiCl₂
L NICI ₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1 4 7 +/- Question 10 of 12 What mass of precipitate (in g) is formed when 52.3 mL of 0.500 M FeCl3 reacts with excess AgNO3 in the following chemical reaction? FeCl3(aq) + 3 AgNO3(aq) → 3 AgCl(s) + Fe(NO3)3(aq) 2 5 8 3 60 9 O g Submit Tap here or pull up for additional resources XU x 100arrow_forwardWhat volume (in mL) of 0.300 M HCl would be required to completely react with 6.25 g of Al in the following chemical reaction? 2 Al(s) + 6 HCl(aq) → 2 AlCl₃ (aq) + 3 H₂ (g)arrow_forwardWhat volume (in L) of 0.246 M HNO3 solution is required to react completely with 38.6 mL of 0.0515 M Ba(OH)2?Ba(OH)2 + 2HNO3 → Ba(NO3)2 + 2H2Oarrow_forward
- How many grams of Zn are required to react with 447.7 mL of 0.202 M HCl? Zn (s) + 2 HCl (aq) → ZnCl2 (aq) + H2 (g)arrow_forwardWhat quantity in moles of precipitate will be formed when 100.0 mL of 2.00 M NaI is reacted with 80.0 mL of 0.900 M Pb(NO₃)₂ in the following chemical reaction?2 NaI(aq) + Pb(NO₃)₂(aq) → PbI₂(s) + 2 NaNO₃(aq)arrow_forwardWhat quantity in moles of precipitate will be formed when 213.0 mL of 0.250 M LiCI is reacted with excess Pb(NO3)2 in the following chemical reaction? 2 LiCI(aq) + Pb(NO3)2 (aq) →PbCl2 (s) + 2 LINO3(aq)arrow_forward
- A person's blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with a potassium dichromate solution. The balanced equation is 16 H*(aq) + 2 Cr20,2-(aq) + C2H5OH(aq) → 4 Cr3+(aq) + 2 CO2(g) + 11 H20(1) If 30.29 mL of 0.05910 M Cr2072- is required to titrate 27.93 g plasma, what is the mass percent of alcohol in the blood plasma? 4.0 0.147 % Submit Answerarrow_forwardWhat quantity in moles of precipitate will be formed when 56.4 mL of 0.300 M AgNO3 is reacted with excess Cal2 in the following chemical reaction? 2 AgNO3 (aq) + Cal2 (aq) → 2 Agl (s) + Ca(NO3)2(aq)arrow_forwardWhat quantity in moles of precipitate will be formed when 53.9 mL of 0.300 M AgNO₃ is reacted with excess CaI₂ in the following chemical reaction? 2 AgNO₃ (aq) + CaI₂ (aq) → 2 AgI (s) + Ca(NO₃)₂ (aq)arrow_forward
- What volume (in mL) of 0.150 M HCl would be required to completely react with 2.90 g of Al in the following chemical reaction? 2 Al(s) + 6 HCl(aq) → 2 AlCl₃ (aq) + 3 H₂(g)arrow_forwardIf 785 mL of 0.350 M AgNO₃ is added to 475 mL of 0.250 M CaBr₂, how many moles of AgBr precipitate will be formed? The balanced equation is 2 AgNO₃(aq) + CaBr₂(aq) → 2 AgBr(s) + Ca(NO₃)₂(aq)arrow_forwardWhat volume (in mL) of 0.300 M HCl would be required to completely react with 5.40 g of Al in the following chemical reaction? 2 Al(s) + 6 HCl(aq) → 2 AlCl₃ (aq) + 3 H₂ (g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY