
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
From the data in the table, determine which of the following fuels produces the greatest amount of heat per gram when burned under standard conditions: CO(g), CH4(g), or C2H2(g).
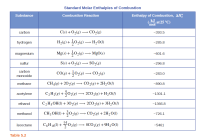
Transcribed Image Text:Standard Molar Enthalpies of Combustion
Substance
Combustion Reaction
Enthalpy of Combustion, AH:
kJ at 25 °C)
mol
carbon
C(s) + O2(8) → CO2(g)
-393.5
hydrogen
H,(g) + 0,(g) → H, O()
-285.8
magnesium
Mg(s) + 0,(3) → MgO(s)
-601.6
sulfur
S(s) + O2(g) → SO2(g)
-296.8
carbon
CO(g) + 0,(g) → CO,(g)
-283.0
monoxide
methane
CH4(8) + 202(8) → CO2(8) + 2H2O(1)
-890.8
acetylene
C2H2(g)+ 02(8) –→ 2C0,(g) + H2O(1)
-1301.1
C,H5OH(1) +30,(8) → 2CO,(g)+ 3H,O(I)
ethanol
-1366.8
methanol
CH3 OH(1) +
CO2(g) + 2H2O(1)
-726.1
C3H18(1) + 02(3) –→ 8CO,(g) + 9H2O(!)
isooctane
-5461
Table 5.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 1.200 g sample of ZnCO3 (s) dissolved in 40.0 mL of a dilute HCl solution (density = 1.10 g/mL) in a coffee cup calorimeter, producing CO2 (g), liquid water and ZnCl2 (aq). The temperature of the solution changes from 24.32 oC to 25.49 oC. The resulting solution has a specific heat of 4.18 J/goC. What would be a balanced equation for this reaction.arrow_forwardAs a system increases in volume, it absorbs 52.0 J of energy in the form of heat from the surroundings. The piston is working against a pressure of 0.597 atm. The final volume of the system is 58.6 L. What was the initial volume of the system if the internal energy of the system decreased by 106.2 J? Volume = Larrow_forwardSucrose is common sugar. It is produced naturally in plants, from which table sugar is refined. A sample of sucrose (C12H22O11) with a mass of 2.68 g is burned in a bomb calorimeter. The heat capacity of this calorimeter is 8.91 kJ/°C. The temperature changes from 23.0°C to 30.5°C. Calculate the heat of combustion of sucrose in kilojoules per mole. Question options: a. -8.54 x 103 b. 4.64 x 105 c. 2.01x 10-4 d. -1.88 x 102arrow_forward
- When 1.836 grams of sucrose (Molar mass 342.3 g/mol) is burned in a bomb calorimeter, the temperature of the calorimeter increases from 22.41°C to 26.63°C. If the heat capacity of the calorimeter is 4.900 kJ/°C, what is the heat of combustion of sucrose?arrow_forwardA 3.59°C increase in temperature occurs when a 0.232 g sample of benzoic acid (C7H6O2) is combusted in a bomb calorimeter. A 1.81 °C increase in temperature occurs when a 0.303 g sample of citric acid (C6H8O7) is combusted in the same bomb calorimeter. The heat of combustion of benzoic acid is -26.37 kJ/g. What is the molar heat of combustion of citric acid? Reminder: Any heats of combustion will be negative since these are exothermic reactions.arrow_forwardA gaseous fuel mixture contains 23.2% methane (CH4), 40.8% ethane (C₂H6) and the rest propane (C3H8) by volume. Part A When the fuel mixture contained in a 1.55 L tank, stored at 756 mmHg and 298 K, undergoes complete combustion, how much heat is emitted? (Assume that the water produced by the combustion is in the gaseous state.) Express your answer with the appropriate units. μA Value Units Review | Constants | Periodic Table ?arrow_forward
- The flame in a torch used to cut metal is produced by burning acetylene (C2H2)(26.04 g/mol) in pure oxygen. Assuming the combustion of 1 mole of acetylene releases 1251 kJ of heat, what mass of acetylene is needed to cut through a piece of steel if the process requires 22.5 × 104 kJ of heat? 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g) ΔH = –2502 kJarrow_forwardWhen a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. In the laboratory a general chemistry student finds that when 3.95 g of CuCl2(s) are dissolved in 108.60 g of water, the temperature of the solution increases from 23.05 to 26.27 °C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.86 J/°C. Based on the student's observation, calculate the enthalpy of dissolution of CuCl,(s) in kJ/mol. Assume the specific heat of the solution is equal to the specific heat of water. AHdissolution kJ/molarrow_forwardA 44.0 g sample of unknown metal at 99.0°C was placed in a constant-pressure calorimeter containing 80.0 g of water at 24.0°C, the final temperature of the system was found to be 28.4°C. Calculate the specific heat of the metal.arrow_forward
- Part A A silver block, initially at 55.8 °C, is submerged into 100.0 g of water at 24.1°C in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 26.3 °C. The specific heat capacities for water and silver are = 4.18 J/(g ·°C) and 0.235 J/(g·°C). What is the mass of the silver block? Express your answer to two significant figures and include the appropriate units. Cs, water • View Available Hint(s) Cs, silver = HA ? Value Units m =arrow_forwardMany barbeque grills use propane gas, C3H8(g) as a fuel source. Using standard enthalpies of formation, calculate the quantity of heat produced, in kJ, when 25 liters of liquid propane is completely combusted in air. (The density of liquid propane is 582 kg/m3.) Note: The propane is in the condensed liquid state in the tank but changes to the gas phase as it is released. Calculate the change in enthalpy for the reaction assuming all reactants and products are in the gas phase.arrow_forwardConsider the change in the internal energy of a system. what is the change in the internal energy, in joules, of a system that does 4.75 x 10^5 J of work, while 3.05 x 10^6J of heat is transferred into the system and 8.25 x 10^6J of heat is transferred from the system to the environment?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY