
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
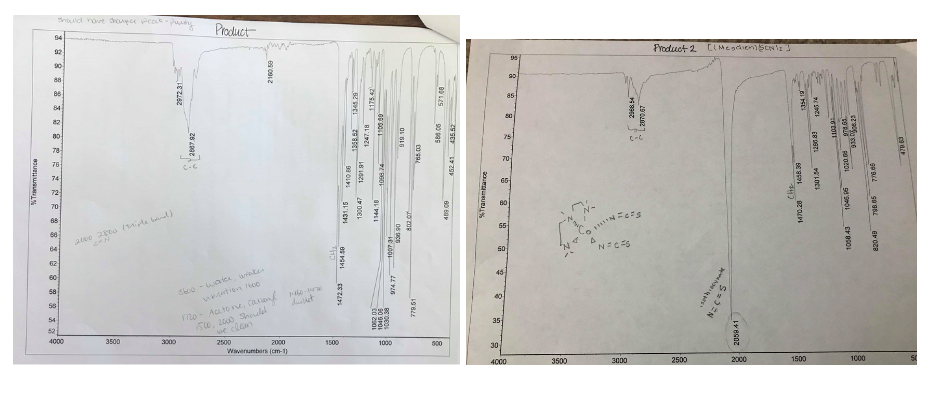
Starting product was [(Me5dien)CoCl2] and reacted with NaSCN to produce [(Me5dien)Co(SCN)2]
Using the graphs attached, the IR data tells you that the binding mode is N coordination. How does the IR data agree (or disagree) with the peak shifts (Uv-Vis spect)
IR
Product 1, [(Me5dien)CoCl2]
29041.04, 2812.76, 2761.62 cm-1 (carbon bond)
1457.35 cm-1 (CH2)
1263.91 cm-1 (possibly C-N stretch)
Product 2, [(Me5dien)Co(SCN)2]
2968.54-2870.67 cm-1 (C-C)
2059.41 cm-1 (N=C=S)
1470.28 cm-1 (CH2)
Uv-vis
[(Me5dien)CoCl2]
Peak A: 531.8 nm, 0.722 Abs
Peak B: 609.7 nm, 0.666 Abs
[(Me5dien)Co(SCN)2]
Peak A: 510.20 nm, 0.357 Abs
Peak B: 608.20 nm, 0.401 Abs
Transcribed Image Text:Sruld vovt doup Peat-Pu
Product
Froduct 2 CIMegdienSeNIz
84
a2
80
80
55
Seoo -watc wroke
wtion op
45
Hle-r
40
mo- Accto ne, CRAan
e cln
2500
Wawenumbers (cm-1)
35
4000
3500
3000
2000
1500
1000
500
3500
300
2500
2000
1500
1000
NTranemttance
2972.31
THE
1454 50
1472.33
9L'LEr
1410.66
1300.47
1247.18
1144.18
1105.89
1030.38
1007 31
974.77
19.10
06 pC6
NEc=5
205841
'LOCI
033.0e.23
820 49
![0.5
0.8
[(Me5dien)Co(SCN)2]
A
[(Me5dien)CoCI2]
0.4
0.6
0.3
0.4
0.2
02
0.1
500
700
900
s00
700
900
Wavelength (nm)
Wavelength (nm)
sqv
sqv](https://content.bartleby.com/qna-images/question/965d603f-c43a-423b-9a04-728914227b3d/f49a2918-41ee-4802-ba77-a4b60da1a5c8/ki3go36.png)
Transcribed Image Text:0.5
0.8
[(Me5dien)Co(SCN)2]
A
[(Me5dien)CoCI2]
0.4
0.6
0.3
0.4
0.2
02
0.1
500
700
900
s00
700
900
Wavelength (nm)
Wavelength (nm)
sqv
sqv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q2/ Calculate the activation energy in( ev /atom) for vacancies formatiom of ( Al ) has ( 7.57 x 10") ( vacancy per m') at 500 e, atomic weight of (26.98) g/m, density of (2.62) g/em', (Vc) of (1) , avagadro number of (6.022 x 10), K of ( 8.62 x 10 ) ev/ atom-karrow_forwardDraw the complex splitting diagram for the indicated hydrogen and determine the expected splitting pattern (dd, dt, ddq, etc.). H u H Harrow_forwardn which of the following compounds the magnetic moment is zero? (28Ni, 26Fe, 24Cr) A. Na3 [FeF6] B. [Cr (H2O) 6] SO4 C. K4 [Fe (CN) 6] D. [Ni (NH3) 6] Cl2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY