
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
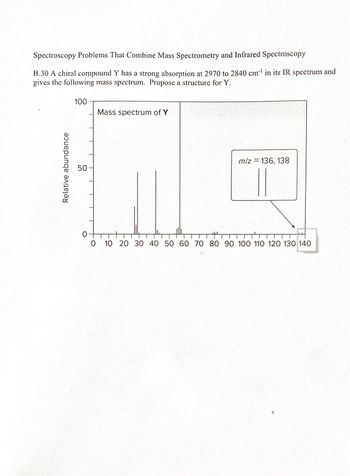
Transcribed Image Text:Spectroscopy Problems That Combine Mass Spectrometry and Infrared Spectroscopy
B.30 A chiral compound Y has a strong absorption at 2970 to 2840 cm¹ in its IR spectrum and
gives the following mass spectrum. Propose a structure for Y.
100
Mass spectrum of Y
Relative abundance
50
m/z = 136, 138
0
0 10 20 30 40 50 60 70 80 90 100 110 120 130 140
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Analyze the 13C NMR spectrum for the C5H1CI compound shown below and determine a structure. Assign all the carbons. Chemical Shift(ppm) Multiplicity 57.3 triplet 33.1 singlet 27.3 quartetarrow_forwardDetermine the formula of the hydrocarbon compound where m/z=134. The mass spectrum indicates an M+2 peak in a 3:1 ratio. The IR spectrum also indicates a strong peak at 1760 cm1 Answer:arrow_forwardThe IR and mass spectra of a compound are shown below. Suggest a possible structure for the compound, explaining how you reach your decision.arrow_forward
- the mass spectrum, IR and 13 C and 1 HNMR spectra for an unknown organic molecule. Determine the structure ofthe molecule.arrow_forwardRelative Intensity 100 CH3 (CH₂)3CH3 LI 40 80 40 20 0++ MS-IW-3004 10 15 20 25 30 35 Using this MS, what is the molecular weight of this compound O 71 O 73 45 50 55 m/z O 72 O There is not enough information 60 65 70 75arrow_forwardDetermine structure. Ignore the 77 ppm in the Carbon 13 NMR. Atomic Mass of structure is 87 garrow_forward
- Using the mass spectrum below propose a structurearrow_forwardI need help with analyzing thisarrow_forwardDetermine the compound (name or structure) from the data. Explain features from each data. Molecular formula: C6H5Br Use molecular formula to determine IHD IR: Identify the presence/absence of five key functional groups NMR: Analyze this last. Consider multiplicity and peak area to confirm compound. The 5 H peak area covers both peaks (at 7.1 and 7.5 ppm Structure?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY