
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Jj.63.
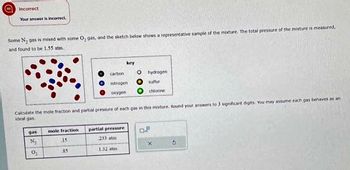
Transcribed Image Text:Incorrect
Your answer is incorrect
Some N₂ gas is mixed with some O, gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured.
and found to be 1.55 atm.
gas
N₂
0₂
mole fraction
15
carbon
85
Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to 3 significant digits. You may assume each gas behaves as an
ideal gas
nitrogen
oxygen
key
partial pressure
233 atmi
1.32 stm
O
O
hydrogen)
Sulfur
chlorine
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider these four gas samples, all at the same temperature. The larger boxes have twice the volume of the smaller boxes. Rank the gas samples with respect to: (a) pressure, (b) density, (c) average kinetic energy, and (d) average molecular speed. (Green spheres are He; violet spheres are Ne.)arrow_forward2. The volume of a gas sample is 235 mL at a temperature of 25 ℃. At what temperature would that same gas sample have a volume of 310. mL, if the pressure of the gas sample is held constant? −47.0 ℃ 69.4 ℃ 33.1 ℃ 120.℃arrow_forwardPlot the data given in Table 5.3 for oxygen at 0C to obtain an accurate molar mass for O2. To do this, calculate a value of the molar mass at each of the given pressures from the ideal gas law (we will call this the apparent molar mass at this pressure). On a graph show the apparent molar mass versus the pressure and extrapolate to find the molar mass at zero pressure. Because the ideal gas law is most accurate at low pressures, this extrapolation will give an accurate value for the molar mass. What is the accurate molar mass?arrow_forward
- 39 A sample containing only NO2 and SO2, has a total pressure of 120. torr. Measurements show that the partial pressure of NO2 is 43 torr. 1f the vessel has a volume of 800.0 mL and the temperature is 22.0°C, how many moles of each gas are present?arrow_forwardIn an experiment in a general chemistry laboratory, a student collected a sample of a gas over water. The volume of the gas was 265 mL at a pressure of 753 torr and a temperature of 27 C. The mass of the gas was 0.472 g. What was the molar mass of the gas?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning