
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
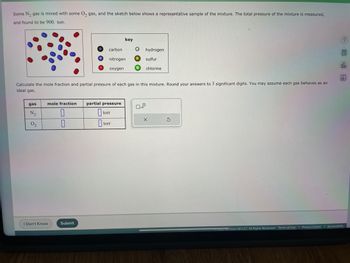
Transcribed Image Text:**Educational Content on Gas Mixture Calculation**
Some \( \text{N}_2 \) gas is mixed with some \( \text{O}_2 \) gas, and the sketch below shows a representative sample of the mixture. The total pressure of the mixture is measured and found to be 900 torr.
**Diagram Explanation:**
- The diagram shows a collection of gas particles.
- A key is provided:
- Black: Carbon
- Blue: Nitrogen
- Red: Oxygen
- White: Hydrogen
- Yellow: Sulfur
- Green: Chlorine
- In this context, focus on the blue points (Nitrogen) and red points (Oxygen) in the sample sketch, as they represent \( \text{N}_2 \) and \( \text{O}_2 \) respectively.
**Calculation Task:**
Calculate the mole fraction and partial pressure of each gas in this mixture. Round your answers to three significant digits. Assume each gas behaves as an ideal gas.
**Table for Input:**
| Gas | Mole Fraction | Partial Pressure (torr) |
|-------|---------------|-------------------------|
| \( \text{N}_2 \) | | |
| \( \text{O}_2 \) | | |
Please enter your calculations in the appropriate boxes.
**Actions:**
- To complete the exercise, input the mole fraction and partial pressure for \( \text{N}_2 \) and \( \text{O}_2 \) in the table.
- Use the "Submit" button to check your answers or click "I Don't Know" if you need assistance.
Consider the assumption that the gases behave ideally to simplify the calculations based on the total pressure given.
---
This educational guide aims to enhance understanding of gas mixtures using the ideal gas law and partial pressure concepts.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 2.65 g sample of an unknown gas at 21 °C and 1.10 atm is stored in a 1.05 L flask. What is the density of the gas? density: g/L What is the molar mass of the gas? molar mass: g/molarrow_forward12. The density of a gas is 4.37 grams/Liter at 72 °C and 1.33 atm. What is the molecular weight of the gas? 000О 80 142 63 93 32arrow_forwardA container initially holds 0.385 moles of a particular gas with an initial volume of 1.78 L. If the amount of gas changes to 0.826 moles, calculate the new volume in L.arrow_forward
- A 3.35 g sample of an unknown gas at 79 °C and 1.00 atm is stored in a 2.15 L flask. What is the density of the gas? density: g/L What is the molar mass of the gas? molar mass: g/molarrow_forwardA gas mixture contains 84% nitrogen and 16% oxygen. If the total pressure is 1.04 atm, what are the partial pressures of each component?arrow_forwardSulfur hexafluoride gas is collected at 3.0 °C in an evacuated flask with a measured volume of 30.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.270 atm . Calculate the mass and number of moles of sulfur hexafluoride gas that were collected. Round your answer to 3 significant digits. mass: mole: molarrow_forward
- A 10.00 L tank at 12.8 °C is filled with 19.2 g of carbon dioxide gas and 15,7 g of dinitrogen monoxide gas, You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. carbon dioxide dinitrogen monoxide mole fraction 0 0arrow_forwardPlease don't provide handwritten solutionarrow_forwardA mixture of hydrogen and xenon gases, in a 6.78 L flask at 18 °C, contains 0.426 grams of hydrogen and 21.5 grams of xenon. The partial pressure of xenon in the flask is__ atm and the total pressure in the flask is__ atm.arrow_forward
- A gas sample containing only BF3, SO2 and S2F2 has the following mass percent composition: 44.3% BF3 and 13.3% SO2. Calculate the partial pressure (torr) of SO2 if the total pressure of the sample is 1.90 atm.arrow_forwardA 3.85 g sample of an unknown gas at 73 °C and 1.05 atm is stored in a 1.35 L flask. What is the density of the gas? density: g/L What is the molar mass of the gas? molar mass: g/molarrow_forwardA 7.00 L tank at 12.4 °C is filled with 19.4 g of sulfur tetrafluoride gas and 10.3 g of dinitrogen difluoride gas. You can assume both gases behar gases under these conditions. Calculate the mole fraction of each gas. Round each of your answers to 3 significant digits. gas mole fraction sulfur tetrafluoride dinitrogen difluoridearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY