
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
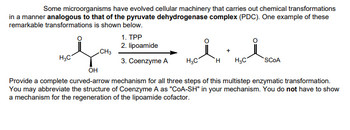
Transcribed Image Text:Some microorganisms have evolved cellular machinery that carries out chemical transformations
in a manner analogous to that of the pyruvate dehydrogenase complex (PDC). One example of these
remarkable transformations is shown below.
معلم
H3C
1. TPP
2. lipoamide
CH3
+
3. Coenzyme A H3C
H
H3C
SCOA
он
Provide a complete curved-arrow mechanism for all three steps of this multistep enzymatic transformation.
You may abbreviate the structure of Coenzyme A as "COA-SH" in your mechanism. You do not have to show
a mechanism for the regeneration of the lipoamide cofactor.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The glycerol carbonylation reaction with CO₂ to produce glycerol carbonate (Figure 1) is thermodynamically hindered due to several reasons including the presence of water as a by-product and the stability of CO2 as a co-reactant. Discuss the possible approach to overcome the issues and be able to produce a higher yield of targeted glycerol carbonate. 0 OH 0 Cat. A HO LOH + CO₂ + H₂O HO. Figure 1arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forward19) What is the final product of the following Hofmann degradation reaction? What is the structure and general name of the intermediate that is involved? H3C NaOH / H,O / Br, H3C `NH2arrow_forward
- Fill in the missing products and intermediatesarrow_forwardFor the following reaction, draw all of the synthetic intermediates that are formed in each step, including the final product.arrow_forward6. For the following reaction, draw all of the synthetic intermediates that are formed in each step, including the final product. 1. H2SO4 2. mCPBA 3. MgBr-CH2CH3 4. H20 HO.arrow_forward
- OCH3 + CH3NH2 a. Draw the structure of the tetrahedral intermediate INITIALLY-FORMED in the reaction shown. • • • You do not have to consider stereochemistry. Do not include counter-ions, e.g., Na+, I, in your answer. In cases where there is more than one answer, just draw one. √n [ ? ChemDoodle b. Draw the structures of the organic products of the acyl transfer reaction. • You do not have to consider stereochemistry. • Draw the neutral form of the products: no charges.arrow_forwardSelect the correct IUPAC name for the following organic substrate, including the R or S designation where appropriate, and draw the major organic product(s) for the SN1 reaction. Include wedge-and-dash bonds and draw hydrogen on a stereocenter. Br HBr HO. Brarrow_forwardFor the following reaction, draw all of the synthetic intermediates that are formed in each step, including the final product.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY