
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
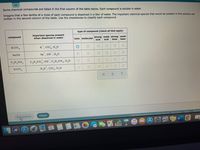
Transcribed Image Text:Some chemical compounds are listed in the first column of the table below. Each compound is soluble in water.
Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The important chemical species that would be present in this solution are
written in the second column of the table. Use the checkboxes to classify each compound.
type of compound (check all that apply)
important species present
when dissolved in water
compound
ionic molecular strong weak strong weak
base
acid
acid
base
KCIO 4
K, C1o,, H,0
NaOH
Na', он , н,о
C,H,NH,
C,H,NH OH ,C,H,NH,, H,0
H C1O4
H,0", Cio, H,0
Check
Explanation
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibi
W
APR
O00 O
O00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- More than 1 can be selectedarrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). 9- Note: "major" chemical species are those present in concentrations greater than 10 major species present when dissolved in water compound formula ロロ fructose H. magnesium sulfate *osân nitrous oxide O'N Ex anation Check O 2022 McGraw Hill LLC. AllI Rights Reserved. Terms of Use | Privacy Center Accessibility 5:24 PM 4/8/202 O -5 0 1 ở H L F11 Delete F4 F5 F10 F12 PrtScr Insert E3 画arrow_forward"Rubbing alcohol" is a mixture that is 70.% isopropyl alcohol (C3H8O) in water. The mixture has a density of 0.79 g/mL at 20°C. What is the concentration of the isopropyl alcohol in molarity? The molar mass of isopropyl alcohol is 60.11 g/mol. a. 11.6 M b. 13.1 M c. 9.2 M d. 30.7 Marrow_forward
- Which one of the following lists three soluble salts? MgSO4,Mg(OH)2,MgS CuSO4,CuS,CuCl2 AgNO3,AgCl,Ag2SO4 lead(II) nitrate, lead(IV) chloride, lead(IV) sulfate iron(II) bromide, iron(III) sulfate, iron(II) hydroxidearrow_forwardA 50.0 mL sample of aqueous sodium hydroxide is titrated with 0.425 M sulfuric acid, and 57 mL are required to reach the equivalence point. Write the balanced chemical equation for this reaction. Answer: A 50.0 mL sample of aqueous sodium hydroxide is titrated with 0.425 M sulfuric acid, and 57 mL are required to reach the equivalence point. What is the molarity of the initial sodium hydroxide solution? Hint: You will need the balanced chemical equation you provided as your answer to the previous question. Report your answer to the correct number of significant figures and with the correct units. Answer:arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 -6 mol/L. major species present when dissolved in water compound formula 0.. nickel(II) chloride NiCl, iron(II) iodide Fel, acetonitrile CH, CN Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center| Accessibi W DELL Delete PgUp F12 PrtScr Insert F9 F10 F11 F6 F7 F8 "R 55arrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound iron(II) iodide ammonium chloride sodium nitrate formula Fel NHẠCI NaNO, major species present when dissolved in water Fe, 1 Na, N, O X mol/L. 0.0 3arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound sodium cyanide copper(II) sulfate nickel (II) chloride formula NaCN CuSO4 NiCl₂ major species present when dissolved in water 0 X mol/L. Śarrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 -9- mol/L. dlo major species present when dissolved in water compound formula glycerol C,H,O, isopropanol C,H,0 nickel(II) chloride NiCl,arrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). -6 Note: "major" chemical species are those present in concentrations greater than 10 mol/L. major species present when dissolved in water compound formula 0,0,.. nickel(II) chloride NiCl, sodium nitrate NaNO3 nickel(II) iodide Nil,arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). Note: "major" chemical species are those present in concentrations greater than 10 mol/L. compound methanol fructose iron(II) iodide formula CH₂OH C6H12O6 FeL₂ major species present when dissolved in water 0 X 0,0,... Śarrow_forwardIdentify if each of the following substance would be classified as an electrolyte or non-electrolyte. Then, identify if each substance would undergo dissociation or dispersion when it is added to water. Substance NH4Cl H₂O₂ CH₂Cl₂ Electrolyte/Non-electrolyte Dissociation/Dispersion A Chemistry teacher is preparing solutions for a classroom activity. For the scenarios below, identify whether the system is undergoing an exothermic change or an endothermic change. When 5.00 g of ammonium nitrate is dissolved in distilled water, the temperature of the solution decreases. When a 5.00 g of sodium hydroxide is dissolved in distilled water, the temperature of the solution increases.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY