
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Some amount of heat energy is removed from a 9cm X 26cm X 46cm block of ice to cool from 0ºC to -26ºC. (Hint: to find mass, use the relation between, density, mass and volume)
Calculate the following:
a) The mas of ice cube in grams
(density of ice = 920 kg/m3).
b) The temperature difference in kelvin
b) The energy removed from ice in calories
. (specific heat of ice = 2093 J/kgºC)
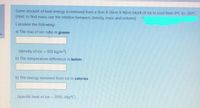
Transcribed Image Text:Some amount of heat energy is removed from a 9cm X 26cm X 46cm block of ice to cool from 0°C to -26°C.
(Hint: to find mass, use the relation between, density, mass and volume)
Calculate the following:
a) The mas of ice cube in grams
en
(density of ice = 920 kg/m³).
!!
b) The temperature difference in kelvin
b) The energy removed from ice in calories
(specific heat of ice = 2093 J/kg°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 25 The temperature at state A is 20°C, that is 293 K. What is the heat (Q) for process A to D, in MJ (MegaJoules)? (Hint: What is the change in thermal energy and work done by the gas for this process?) Your answer needs to have 2 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement. p (atm) 5 4 3 2 1 O A D 1 1 2 3 4 B I 5 → V (m³)arrow_forwardA 0.03 kg lead bullet traveling 99 m/s strikes an armor plate and comes to stop. If all of the bullet’senergy is converted to heat that it alone absorbs, what is its temperature change? Specific Heat Capacity of the lead is g = 9.8 m/s2 Calculate to one decimal.arrow_forward#4. The rate of costing. of as dT dt where T = Q body fig. below, can be expressed = -k (T- Ta) : temperature of the body (ĉ). Te = temperature of the surrounding medium (2) and k = 4 proportiona- 2ty constant (per minste). Thus, this equation (Called Newton's law of Cooling) specifies that the vate of cooling is proportional to the difference in the temperature of the body and of the surrounding medium. a metal ball heated to 80 °C is dropped into water that is held constant at Ta = 20°C, the temperature of the ball changes. as in Time, min 0 5 10 15 20 25 T₁ °C 80 44.5 30.0 2411 21.7 2017 wtilize numerical differentiation to determine dI at each Value of time. plot dI Versus T-Ta and dt employ linear regression to ellalvate k. dtarrow_forward
- How much heat Q would be needed to completely evaporate 37.5 g of boiling water at a temperature of 100 °C? Express the answer in units of joules. The latent heat of vaporization for water at 100 °C is 2260 J/g. Q =arrow_forwardInstructions: Provide the answer in standard scientific notation with SI units that do not have prefixes (except for kg). Provide final answer with correct units and 3 significant figures. Please avoid round off errorarrow_forwardA 2 kg. block of copper is removed from a freezer where its temperature was maintained at -13 Co . How much heat does the copper absorb as it is warmed to -5.2 Co ? (The specific heat capacity of copper is 390) Calculate answer to one decimal.arrow_forward
- 4. 100.0 g of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4180J/kg°C, calculate the amount of heat energy needed to cause this rise in temperature. 5. A 0.3 kg piece of copper is heated and fashioned into a bracelet. The amount of energy transferredby heat to the copper is 66,300 J. If the specific heat of copper is 390 J/kg celcius, what is the change of thecopper's temperature?arrow_forwardMethanol ( density = 787 kg/m³) is used to cool an engine. If 0.03 m3 of coolant is used and the cooling process results in a change in internal energy of 912 kJ, what is the change in temperature of the coolant (deg C)? Report your answer to 2 decimal places.arrow_forwardIf mass of air in a house is 278.6376kg. And the specific heat of air is 1.0kj/kgK. How much heat energy is needed to heat air by 10K? Use Q=mc∆Tarrow_forward
- How much energy in Joules is required to melt 415g of ice? The latent heat of fusion of ice is: Lf = 3.33 × 10³J/kg Do NOT include units in your answer. Round your answer to 2 decimal places. Do NOT change to scientific notation. Ignore significant figures.arrow_forwardMust give answer then also give answer in significant fig format 11. The coolest part of the sun is the region called the chromosphere, which is the region above the sun's apparent surface, or photosphere. At 1,100 km above the photosphere, the temperature of the chromosphere is about 3,500,K. What is this temperature in degrees Fahrenheit and degrees Celsius?.odarrow_forwardAn unknown hot metal at 148.7°C with a mass of 49.6g was mixed with 28.61g of water at an initial temperature of 19.74°C. A final temperature (for both water and metal) of 27.71°C was reached. In units of J/(g°C), what is the specific heat of the unknown metal? Note: In the space below, please enter you numerical answer. Do not enter any units. If you enter units, your answer will be marked as incorrect.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON