
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
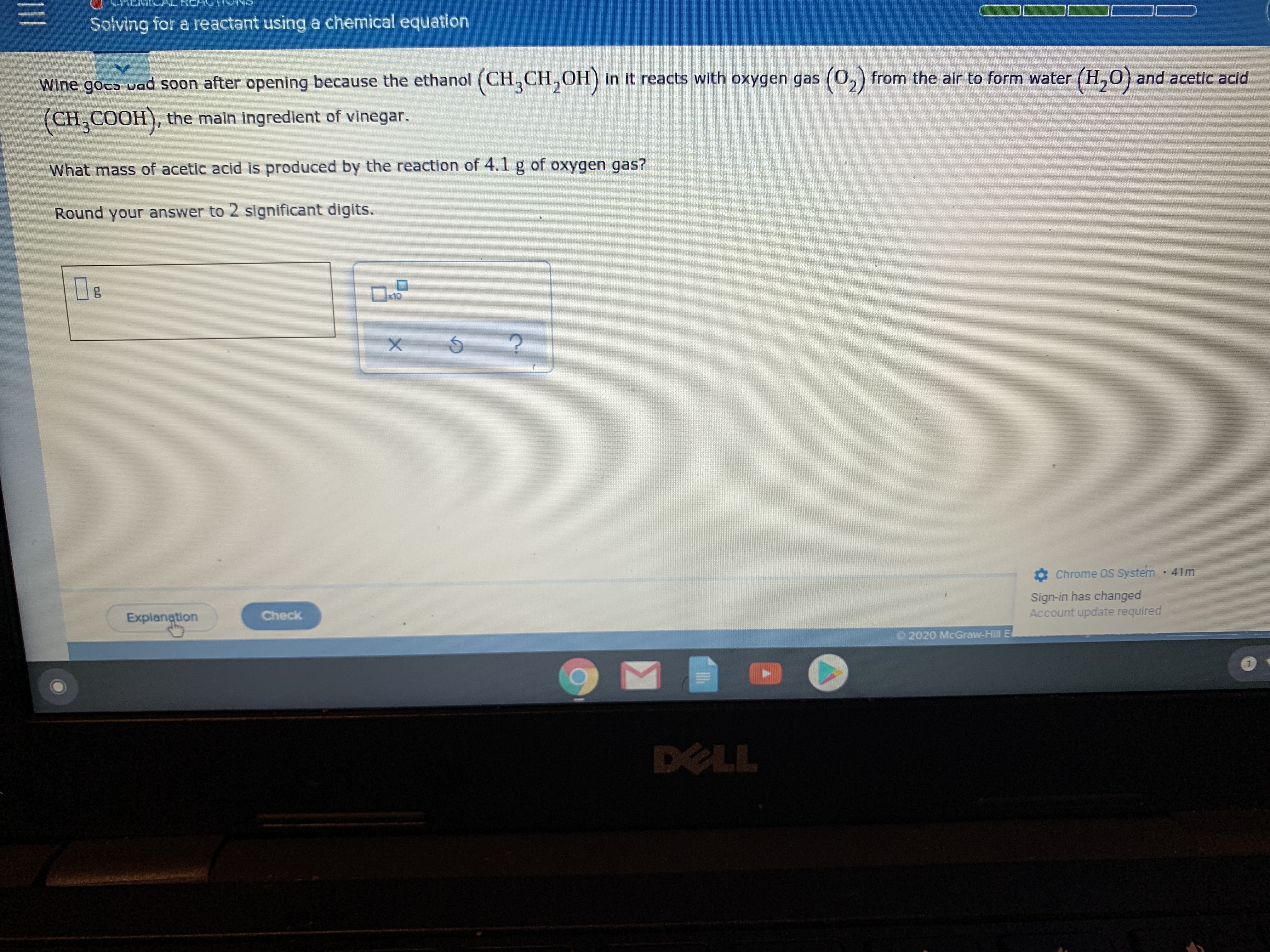
Transcribed Image Text:Solving for a reactant using a chemical equation
Wine goco vad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the alr to form water (H,0) and acetic acid
(0,)
(CH,COOH), the main ingredient of vinegar.
What mass of acetic acid is produced by the reaction of 4.1 g of oxygen gas?
Round your answer to 2 significant digits.
x10
Chrome OS System
41m
Sign-in has changed
Account update required
Explanation
Check
2020 McGraw-Hill E
DELL
11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- 591470&isprv=&drc3D0&qi=2475808&cfql=1&dnb3D08fro= How many moles of Al2(CO3)3 are there in 127 g of Al2(CO3)3 ? Round your answer to 2 decimals. Your Answer: Answer units D Add attachments to support your workarrow_forwardWine goes bad soon after opening because the ethanol CH3CH2OH in it reacts with oxygen gas O2 from the air to form water H2O and acetic acid CH3COOH, the main ingredient of vinegar. What mass of ethanol is consumed by the reaction of 9.7g of oxygen gas? Round your answer to 2 significant digits.arrow_forwardWhat mass of octane is consumed by the reaction of 2.13g of oxygen gas?arrow_forward
- Ammonium phosphate (NH4), PO4) ("HN)) (H; PO,) with ammonia (NH, ). is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid What mass of ammonium phosphate is produced by the reaction of 4.3 g of ammonia? Round your answer to 2 significant digits.arrow_forwardk Please don't provide handwritten solutionarrow_forwardPlease helparrow_forward
- An explosive reaction between butane and oxygen occurs as follows. 2 C4H10 (g) + 13 02 (g) --> 8 CO2 (g) + 10 H2O (g) If 0.625 moles of butane and 3.5 moles of oxygen gas react predict the theoretical yield (in moles) of water vapor that would be produced. moles H20 (g) [round your answer to the correct number of significant figures.] Type your answer... Sarrow_forwardAmmonia NH3 chemically reacts with oxygen gas O2 to produce nitric oxide NO and water H2O What mass of nitric oxide is produced by the reaction of 3.5 g of ammonia? Round your answer to 2 significant digits.arrow_forwardO CHEMICAL REACTIONS Solving for a reactant using a chemical equation OOD 13 Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the air to form water (H,0) and acetic acid (CH,COOH), the main ingredient of vinegar. What mass of oxygen gas is consumed by the reaction of 8.4 g of ethanol? Be sure your answer has the correct number of significant digits. Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acces APR 18 888 DIIarrow_forward
- Ammonium phosphate ((NH4) P04) is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 2.8 g of phosphoric acid? db Round your answer to 2 significant digits.arrow_forwardAmmonium perchlorate (NH4CIO4) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas (N2), chlorine gas (Cl2), oxygen gas (O2), water (H2O) and a great deal of energy, What mass of oxygen gas is produced by the reaction of 4.98 g of ammonium perchlorate? Round your answer to 3 significant digits.arrow_forwardWine goes bad soon after opening because the ethanol (CH3CH₂OH) in it reacts with oxygen gas (O₂) from the air to form water (H₂O) and acetic acid (CH3COOH), the main ingredient of vinegar. What mass of acetic acid is produced by the reaction of 4.38 g of ethanol? Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY