
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
I need help the molarity to show work with this table
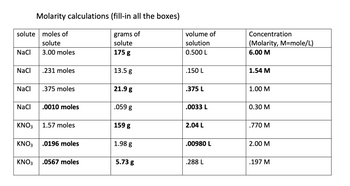
Transcribed Image Text:**Molarity Calculations (Fill-in All the Boxes)**
This table displays calculations of molarity involving different solutes, given their amounts in moles, grams, and the volume of their solutions.
| **Solute** | **Moles of Solute** | **Grams of Solute** | **Volume of Solution (L)** | **Concentration (Molarity, M = mole/L)** |
|------------|---------------------|---------------------|----------------------------|------------------------------------------|
| NaCl | 3.00 moles | **175 g** | 0.500 L | **6.00 M** |
| NaCl | 0.231 moles | 13.5 g | 0.150 L | **1.54 M** |
| NaCl | 0.375 moles | **21.9 g** | 0.375 L | **1.00 M** |
| NaCl | **0.0010 moles** | 0.059 g | 0.0033 L | **0.30 M** |
| KNO₃ | 1.57 moles | **159 g** | 2.04 L | **0.770 M** |
| KNO₃ | **0.0196 moles** | 1.98 g | 0.00980 L | **2.00 M** |
| KNO₃ | **0.0567 moles** | 5.73 g | 0.288 L | **0.197 M** |
**Note:**
- The bold entries represent the information that is given, and the other entries denote the calculated values.
- Molarity is calculated using the formula: \( \text{Molarity} (M) = \frac{\text{moles of solute}}{\text{volume of solution in liters}} \).
- This table serves as a practice for understanding and computing molarity in solution chemistry.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of 0.220 M HBr solution is required to produce 0.0190 moles of HBr? Answer should be in mLarrow_forward11. You have a solution that is 18.2% sodium thiosulfate, Na2S2O3 , by mass. a. What mass of sodium thiosulfate is in 75.0 g of solution? b. How many moles of sodium thiosulfate are in 75.0 g of solution? please show workarrow_forwardd. Why do you not need to know the volume of one drop? Provide a sample calculation showcasing this.arrow_forward
- 14. What mass of barium nitrate is dissolved in 31.29 mL of a 4.78 M solution? please show workarrow_forwardStuck need help! Problem is attached. please view attachment before answering. Really struggling with this concept. Please show all work so I can better understand ! Thank you so much.arrow_forwardAn aqueous potassium iodate (KIO,) solution is made by dissolving 525 grams of KIO, in sufficient water so that the final 3. volume of the solution is 3.60 L. Calculate the molarity of the KIO, solution. [KIO,]= terms of use help, about us careersarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY