
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Solubility (g/100g water)
Question 17
What is the concentration (in molality, m, mol/kg water) in a saturated amonium bromide solution at 40 °C? Use the solubility graph below for your calculation.
091
18'HN
ON)9d
140
120
08
KCIO,
M9SO,
-09
NaCI
CdSeO,
-20-
08
00L
09
Temperature (°C)
O A. 9.2 m
O B. 90. m
OC. 0.92 m
O D. 0.0092 m
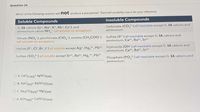
Transcribed Image Text:Question 24
Which of the following reaction will not produce a precipitate? The brief solubility rule is for your reference.
Soluble Compounds
Insoluble Compounds
Carbonate (CO.2-) all insoluble except G. IA cations and
G. IA cations (Li*, Na, K, Rb*, Cs+), and
ammonium cation NH (all soluble no exception)
ammonium.
Nitrate (NO-), perchlorate (CIO,-), acetate (CH COO-)
(all soluble no exception)
Sulfide (S2-) all insoluble except G. IA cations and
ammonium, Ca2+, Ba2+, Sr2+
Hydroxide (OH-) all insoluble except G. IA cations and
ammonium, Ca²+, Ba2+, Sr²+
Halide (F, CI-,Br,I-) all soluble except Ag*, Hg,2*, Pb²+
Sulfate (SO 2-) all soluble except Sr2+, Ba²+, Hg,2+, Pb²*
Phosphate (PO,3-) all insoluble except G. IA cations and
ammonium.
O A. CaCl2 (aq)+ AGNO3(aq)
O B. Nacl(aq)+ Ba(NO3)2(aq)
O C. Na2CO3(aq)+ Mgl2(aq)
O D. KOH(aq)+ Cu(NO3)2(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Determine the number of components in each of the following systems a. CH3COOH + CHẠOH + CH3COOC2Hs + H2O b. Aqueous solution of sodium acetate (NaC₂H5O₂)arrow_forwardCalculate the solubility of CaF2 in water at 25 °C. You'll find K data in the ALEKS Data tab. sp Round your answer to 2 significant digits. 0 -/- L Explanation 80°F Partly sunny Check Q Search LD Ⓒ2023 McGraarrow_forwardest Ort a gamb rement: 56°F Cloudy Sodium chloride (NaCl) has a solubility of 36.0 g/100 g H₂O at 20 °C. Select the temperature at which the solubility of NaCl will be higher than 36.0 g/100 g H₂O among the following. U 20 °C O 10 °C O 40 °C chemistry and reaction of Employ... paymen... O 5 °C receipt % 10 f6 & hparrow_forward
- The molar solubility of Iron(II) hydroxide in a water solution is 30 F3 Submit Answer $ 4 R F % 5 Retry Entire Group 9 more group attempts remaining Cengage Learning Cengage Technical Support F5 G A 6 MacBook Air Y H & 7 A U * 00 8 J ► 11 F8 1 9 ➤➤ K ded for this question. O V 1 0 M. F10 P 1 A Previous F11 { = 1 Next> Save and Exit ? 11arrow_forwardAt 90°C, if 51 g of potassium chloride are completely dissolved, the solution is considered to be SOLUBILITY CURVES KI 140 130 120 100 NONO, KNO3 90. HCI, 70 NH CI 60 KCI 50 40 Naci KCIO, 30 20 10 O 10 20 30 40 50 do 70 80 9o 100 TEMPERATURE °C A. supersaturated B. saturated C. uncool GRAMS OF SOLUTE /100g H,0arrow_forward1 4 g at 15. °C, Calculate the mass of O that's dissolved in 200. mL of a saturated solution of O in hexane at mL A certain organic compound O has a solubility in hexane of 0.601 this temperature. Be sure your answer has the correct unit symbol and 3 significant digits. ロ ロ。 ロ ロロ Submit Assignment O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility II Finder F10 F11 F12arrow_forward
- Paus gnment/takeCovalentActivity.do?locator=assignment-take 国Rei O Jesuit Extreme Oath. O App Academy Open B Free College - Top.. O Robinhood - Print Coinbase - Buy & S. A https://www.e-read.. [References] entration Use the References to access important values if needed for this question. What is the solubility of carbon dioxide (in units of grams per liter) in water at 25 °C, when the CO, gas over the solution has a partial pressure of 0.337 atm? ky for CO, at 25 °C is 3.36x102 mnol/L'atm. g/L Submit Answer Retry Entire Group 9 more group attempts remaining KPravious Email Instructor Save and Exit 9:10 AM 72°F Cloudy 9/10/2021 * 10 brt sc delete 6 8. backspace enter L.arrow_forwardCalculate the solubility of CaF, in water at 25 °C. You'|l find K.n data in the ALEKS Data tab. 2 sp Round your answer to 2 significant digits. x10arrow_forwardKsp(CaF2) = 3.9 x 10-11. Assume that we add enough amount of CaF2 into the following 3 solutions so that CaF2 will become saturated in each solution. Solution A. 0.30 M Ca(NO3)2 (aq). Ca(NO3)2 is highly soluble in water. Solution B. 0.30 M NaF (aq). NaF is highly soluble in water. Solution C. 0.30 M NaNO3 (aq). NaNO3 is highly soluble in water. Which of the following statements is true about the solubility of CaF2 in the above 3 solutions? Choose one option only. Options: a. CaF2 (s) has the lowest solubility in Solution B. b. CaF2 (s) has the highest solubility in Solution B. c. CaF2 (s) has the highest solubility in Solution A. d. CaF2 (s) has the lowest solubility in Solution C. e. CaF2 (s) has the lowest solubility in Solution A.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY