
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
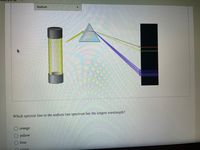
Transcribed Image Text:Sodium
Which spectral line in the sodium line spectrum has the longest wavelength?
orange
yellow
blue
O violet
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An energy of 353 kJ/mol is required to cause a magnesium atom on the metal's surface to lose an electron. Calculate the lowest frequency of light that can ionize a magnesium atom.arrow_forwardWhich has the greater wavelength, blue light or red light? How do the frequencies of blue light and red light compare? How does the energy of blue light compare with that of red light? Does blue light have a greater speed than red light?arrow_forwardion What is the color of light with a frequency of 5.773 x 104 s? Refer to the electromagnetic spectrum as needed. S Determine the frequency and energy for yellow light with a wavelength of 582.8 nm. V = S E = kJ/mol Determine the wavelength and frequency for light with energy of 255.6 kJ/mol. nmarrow_forward
- In order for the transition of an electron in a hydrogen atom to produce light visible to the human eye, what must be the final energy level of the electron? O 7 4 1 O 2arrow_forwardWhich of the following colors from the visible range has the greatest frequency yellow red orange green O blue Which of the following colors from the visible range has the shortest wavelength green red orange O blue O yellowarrow_forwardUsing the Bohr model, pick which electronic transition will emit a photon with the shortest wavelength. Group of answer choices n = 1 → n = 3 n = 2 → n = 1 n = 6 → n = 4 n = 3 → n = 2arrow_forward
- The energy of an electron is based on its quantum numbers that define which characteristics? color charge number of electrons distance from nucleus orientation in space shape For the fourth level (n = 4), the subshells that you would find include 12arrow_forwardConvert between number of photons and energy given a particular wavelength. Low level light therapy (LLLT) using RED light was observed to promote wound healing. The daily dose provided was 24 J/cm. Given the wavelength of 635 nm, how many photons were required to deliver the dose to a 1 cm? area?arrow_forward1. Complete following table for regions of the visible region of the electromagnetic spectrum: Visible Region Wavelength Range (nm) Visible Region Wavelength Range (nm) purple/violet yellow blue orange green redarrow_forward
- Which color of visible light has the highest frequency? Green Yellow Orange Redarrow_forwardFix any errors in these proposed electron configurations. number of electrons in atom 32 20 12 proposed electron configuration 6 10 1s² 1p 2s²³ 2p 2d¹ 35² 3p 6 1s 2s² 2p 3s 3p 3d 5 2 2 8 2 1s 2s 2p Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY