
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
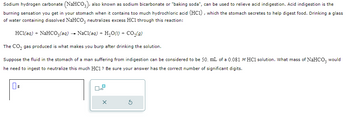
Transcribed Image Text:Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the
burning sensation you get in your stomach when it contains too much hydrochloric acid (HC1), which the stomach secretes to help digest food. Drinking a glass
of water containing dissolved NaHCO3 neutralizes excess HC1 through this reaction:
HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
The CO₂ gas produced is what makes you burp after drinking the solution.
Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 50. mL of a 0.081 M HCl solution. What mass of NAHCO3 would
he need to ingest to neutralize this much HC1? Be sure your answer has the correct number of significant digits.
0
x10
X
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete and balance the following acid-base equations. (Assume that these reactions go to completion. Use the lowest possible whole number coefficients. Include states-of-matter under the given conditions in your answer.) (a) A solution of Mg(OH)2 is added to a solution of HCH3CO2. (b) A solution of HOCl reacts with solid Ni(OH)2. I am a little confused by these equations if you wouldn't mind going into detailarrow_forwardUlat Is 5. Carbon dioxide slowly reacts with water over time, as shown in the equation below. How does this affect the molarity of the NaOH solution over time? Be specific and include a chemical equation to support your answer. CO, (g) + H,0(1)=H,CO,(aq)arrow_forwardTitration of a 20.00 mL sample of acid rain required 4.13 mL of 0.08424 M KOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration (in mol/l and %) of sulfuric acid in this sample of rain?arrow_forward
- using this balanced equation CaCO3(s) + 2 HCl(aq) ----> H2O(l) + CO2(g) + CaCl2(aq) Stomach acid is 0.100 M HCL. An active ingreident found in antacids such as Alka Seltzer is calcium carbonate, CaCO3. Calculate grams of calcium carbonate needed to react with 80 ml of stomach acid.arrow_forwardA sample weighing 1.731g contains a mixture of the triprotic citric acid, H3C6H5O7 and sodium sulfate, Na2SO4. The sample mixture was dissolved in water and then titrated with a 0.3550 M sodium hydroxide solution. It required 28.32mL of the base to completely neutralize the citric acid. What is the percent of sodium sulfate in the mixture?arrow_forwardA 0.261 g sample of NaHC2O4 (one acidic proton) required 17.5 mL of sodium hydroxide solution for complete reaction. Determine the molar concentration of the sodium hydroxide solution.arrow_forward
- A 0.5504-g sample of KHP (potassium hydrogen phthalate, KHC8H4O4; molar mass = 204.22 g/mol) is completely dissolved in enough deionized water to make 50.00 mL of solution. The resulting solution is titrated with a NaOH solution of unknown concentration. If 21.50 mL of the base solution is needed to reach the end-point, what is the molar concentration of NaOH solution? The reaction occurs as follows: NaOH(aq) + KHC8H4O4(aq) --> NaKC8H4O4(aq) + H2O(l) (A) 0.2457 M (B) 0.1254 M (C) 0.05390 M (D) 0.03823 Marrow_forwardA scientist is investigating the solubility of two polyatomic ions, oxalate (C2O2−4C2O42−) and arsenate (AsO3−4AsO43−), which are not listed in the table of solubility guidelines. The scientist starts with four solutions made of water-soluble salts. Solution A contains sodium arsenate. Solution B contains ammonium oxalate. Soution C contains silver chlorate. Solution D contains aluminum bromide. Solution Solute Color of Solution A Na3AsO4Na3AsO4 colorless B (NH4)2C2O4(NH4)2C2O4 colorless C AgClO3AgClO3 colorless D AlBr3AlBr3 yellow The results of mixing each solution in pairs are shown in the table. Experiment Solutions Mixed Result 1 A + B no precipitate, colorless solution 2 A + C brown precipitate 3 A + D white precipitate 4 B + C white precipitate 5 B + D white precipitate 6 C + D yellow precipitate Identify the formula for each precipitate that forms. precipitate from A+C: precipitate from A+D: precipitate…arrow_forwardA chemistry student needs to standardize a fresh solution of sodium hydroxide. He carefully weighs out 30. mg of oxalic acid (H,C,0,), a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250. mL of distilled water. The student then titrates the oxalic acid solution with his sodium hydroxide solution. When the titration reaches the equivalence point, the student finds he has used 62.3 mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution. Round your answer to 2 significant digits. x10arrow_forward
- Which of the following is a balanced equation for a neutralization reaction? O Al(OH)3(s) + HCI ----> 3 H2O + AICI3(aq). O KOH(s)+ HNO3 -> H2O + KNO3(aq) -- Ca(OH)2(s) + HNO3 ---> 2 H2O + Ca(NO3)2(aq) O Mg(OH)2(s) + HNO3 ---> 2 H2O + Mg(NO3)2(aq) O2 Al(OH)3(s) + HCI ---> 3 H2O + AICI 3(aq)arrow_forwardMethanoic acid is also called formic acid. It has the chemical formula HCOOH(l). It is a colourless fuming liquid that is mainly used as a preservative. It exhibits the following equilibrium in water:HCOOH(aq) + H2O(l) → HCOO–(aq) + H3O+(aq) 1) A 4.50 g tablet of magnesium hydroxide neutralizes 400.0 mL of stomach acid, HCl. What is the concentration of HCl in the stomach? PLEASE HELP WITH THIS ITS EXTREMELY URGENTarrow_forwardThe spectator ions in the following are: Mg(OH)2(aq) + 2 HCl(aq) → MgCl2 + 2 H2O 2 H+ and 2 Cl- Mg2+ and 2 (OH)- Mg2+ and 2 Cl- 2 H+ and 2 (OH)-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY