
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
I need help solving this chemistry equation on solving for a reactant in solution.
Transcribed Image Text:+II
P
08
CO
团
R
%#3
A ALEKS - Cameron Hite - Learn
Strong acid solutions (video) | Kh x
M Chem - merprockz@gmail.com - X
d: One way the U.S. Envi X
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf8oMM9svvguKhbGCwZ9KTTwF0JXL6qVOLHEU1dB2W5prXD9BvdYc1k1GK32UsCNOHq?1oB.. 2 ☆
O CHEMICAL REACTIONS
1/3
Cameron
三
Solving for a reactant in solution
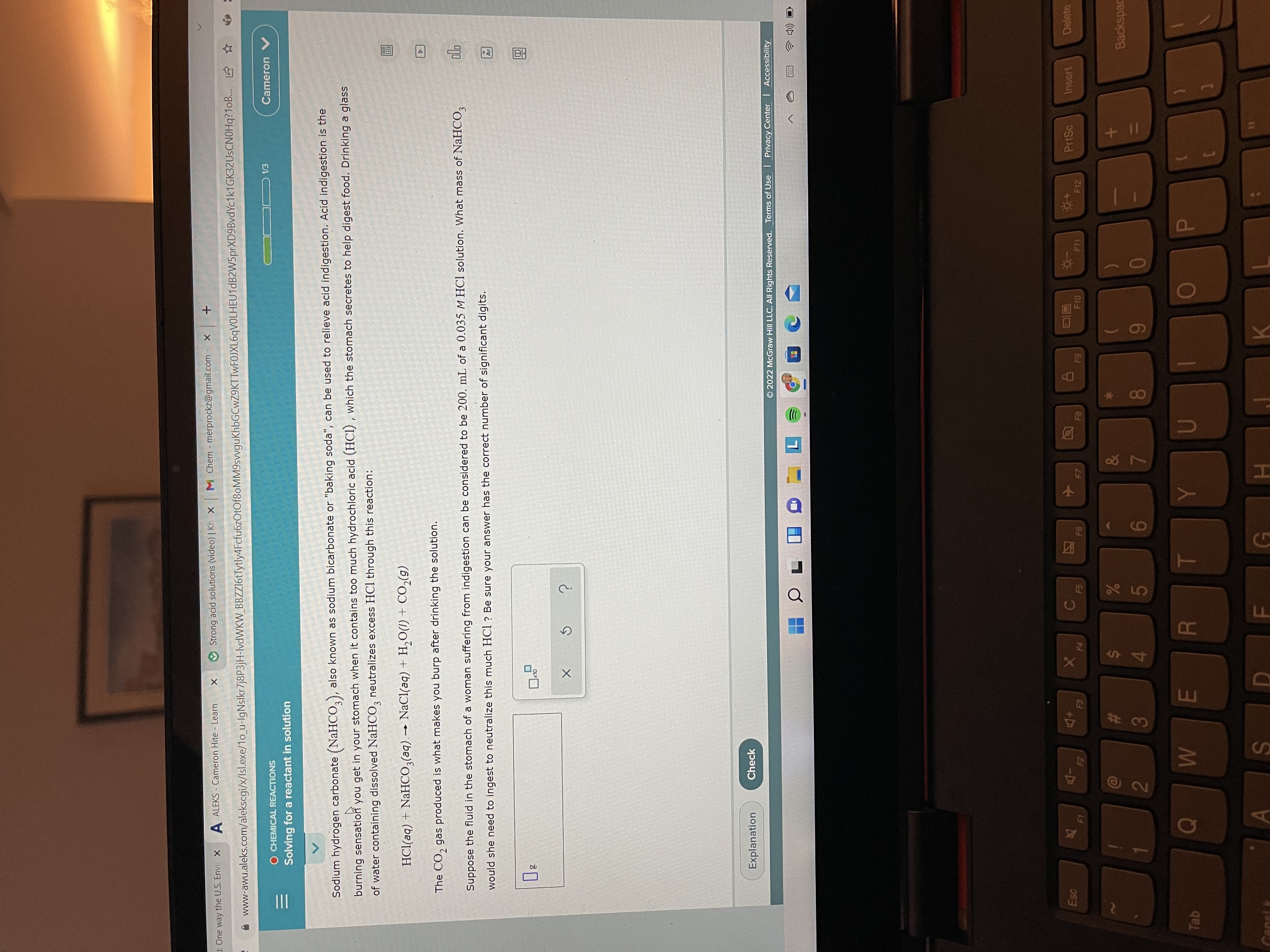
Sodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the
burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI) , which the stomach secretes to help digest food. Drinking a glass
of water containing dissolved NaHCO, neutralizes excess HCl through this reaction:
HCl(aq) + NaHCO,(aq) → NaCl(aq) + H,O(1) + CO,(g)
画
The CO, gas produced is what makes you burp after drinking the solution.
Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200. mL of a 0.035 M HCl solution. What mass of NaHCO,
would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.
Explanation
Check
O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility
Esc
PrtSc
Insert
Delete
-Do
+DD
F8
F10
F11
F12
F4
63
&
%23
Backspac
%24
6
Tab
Cansls
K.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Vrite the equations that rarrow_forwardHow would the following MaxwellI Boltzmann distribution change if temperature were increased? Draw the expected change and explain what is happening to reaction rate. Number Of Molecules EA Energyarrow_forwardCan you explain me how to do the fundamental application of Beer’s Law plot in determining the concentrations of a species involved in Chemical equilibrium.arrow_forward
- The solubility of Mg(OH), in water at 25 °C is measured to be 0.0096 . Use this information to calculate K, for Mg(OH),. sp Round your answer to 2 significant digits.arrow_forwardFor the denitrification [microbiological conversion of nitrite/nitrate (N-containing contaminants) to nitrogen gas (harmless end product)] to be more effective, an external carbon source (e.g., methanol) is frequently added for denitrifiers (denitrifying microorganisms) during the reaction. The following chemical reaction is for when methanol is added for this purpose. Calculate how much (concentration) of methanol (in mg/L) needs to be added to completely convert 30 mg/L of NO3--N to N2 gas. 0.1667 CH3OH + 0.1343 NO3- + 0.1343 H+ ↔ 0.0143 C5H7O2N + 0.06 N2 + 0.3505 H2O + 0.0952 CO2arrow_forwardUse the provided table as a guidearrow_forward
- The addition of acid to the reaction mixture is done drop wise as the process is exothermic. Group of answer choices True Falsearrow_forwardAnswer correctly please!arrow_forwardIncreasing the volume of the gaseous decomposition reaction of carbonic acid into water and carbon dioxide will shift the reaction to the left. True Falsearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY