
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
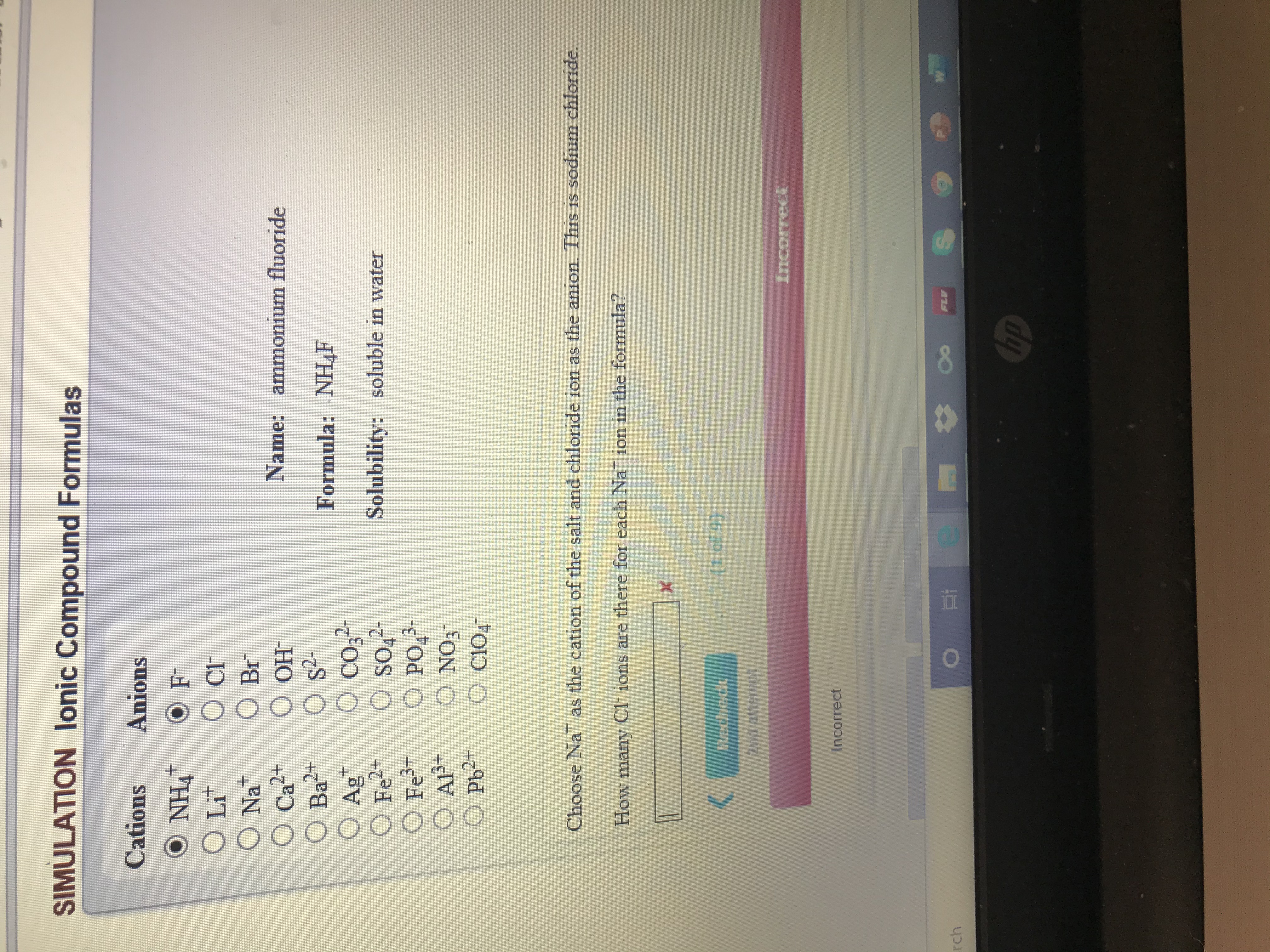
Transcribed Image Text:SIMULATION lonic Compound Formulas
Cations
Anions
O NH4
O Lit
O cF
O Br
O OH
O s?-
O co,
O so,2-
PO,-
O NO3
O C104
Na
Name: ammonium fluoride
O Ca²+
2+
Formula: NHF
O Ag
O Fe2+
O Fe+
O AlP+
O Pb2-
Solubility: soluble in water
Choose Na as the cation of the salt and chloride ion as the anion. This is sodium chloride.
How many CF ions are there for each Na ion in the formula?
(1 of 9)
Recheck
2nd attempt
Incorrect
Incorrect
FLV
rch
Cop
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- ||| V What are the molecular and empirical chemical formulas of a compound made up of these molecules? H H H H | | —C—C—CO-H O ATOMS, IONS AND MOLECULES Understanding the difference between a molecular and empiric... esc H—Ö- H-Ö-c 1 HTCI I H molecular formula: empirical formula: Q Explanation 2 Check W -CIH Н 0 0 #3 E The lines stand for chemical bonds between the atoms. You can ignore the dots you'll learn about them later. C X > $ 4 R S Costa do 5 % 6 MacBook Pro T A 6 G I Y 7 2022 McGraw Hill LLC. All Rights Rese H You 8 U Jarrow_forwardCan you help me with this chemistry questionarrow_forwardGive clear Detailed Solution with explanationarrow_forward
- OWLV2 | Online teachin X According To The Following+ My Home gagenow.com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take&takeAsignmentSessionLocator=assignment-take [Review Topics] [References] E 2req Use the References to access important values if needed for this question. 3 2reg For the following reaction, 0.397 grams of hydrogen gas are allowed to react with 54.1 grams of iodine. S 2req hydrogen(g) + iodine(s) – → hydrogen iodide(g) What is the maximum mass of hydrogen iodide that can be formed? grams What is the FORMULA for the limiting reagent? Es 2req is 2req What mass of the excess reagent remains after the reaction is complete? grams ts 2req ts 2req Submit Answer Retry Entire Group 9 more group attempts remaining ts 2req ots 2req ots 2reg ots 2req ots 2req pts 2req Previor Show Hint a $ hp Ins prt sc f9 12 delete 16arrow_forward4) lonic vs Covalent: If ionic, give ionic formula (showing charges) & if covalent, give structural formula (bonds), then answer if it were a good electrolyte. g) C3F4. vs MgCl2arrow_forwardBr Li Et₂0 Cul I CHCI3 (CH3)3CO-Karrow_forward
- ed Complete the following for the compound ammonium phosphate. formula = (NH4)3PO4 atom nitrogen hydrogen phosphorus oxygen Submit Answer number in formula important values if needed for this question Retry Entire Group 9 more group attempts remainingarrow_forwardplease answer quicklyarrow_forwardOnly typed solutionarrow_forward
- Match the left 'cation' column with the right 'anion' columnto create a formula of a soluble ionic compound. Use each anion and cation only once. Na+ S2- Sr2+ CO32- Co2+ NO-3 Pb2+ SO42-arrow_forwardNonearrow_forward(c) 4.27 M NaH2PO4 lons: O PO43- H2PO4 PO4 3+ Na O Na* NaH* [cation*] = i M [anion] = Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY