
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Watch the Ag video.
youtube.com/watch?v=npqooiE6DhE
Does a chemical change occur? Cite macroscopic (visible to the naked eye) evidence that supports your decision.
Cite microscopic (only can been seen with a microscope) that supports if a chemical change occurred or not.
Ag (s)+HCl (aq) —->
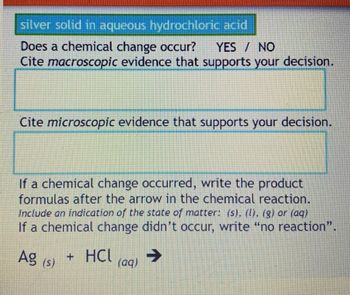
Transcribed Image Text:silver solid in aqueous hydrochloric acid
YES / NO
Does a chemical change occur?
Cite macroscopic evidence that supports your decision.
Cite microscopic evidence that supports your decision.
If a chemical change occurred, write the product
formulas after the arrow in the chemical reaction.
Include an indication of the state of matter: (s), (I), (g) or (aq)
If a chemical change didn't occur, write "no reaction".
➜
Ag (5)
+ HCL
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For the following reaction, 96.8 grams of perchloric acid (HCIO4) are allowed to react with 26.6 grams of tetraphosphorus decaoxide . perchloric acid (HCIO4) ( aq ) + tetraphosphorus decaoxide ( s )- → phosphoric acid ( aq ) + dichlorine heptaoxide (1) What is the maximum amount of phosphoric acid that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? gramsarrow_forwardGiven the following unbalanced equation, select the correct net ionic equation. CaCO3(s) + HCI(aq) CaCl2(aq) + H20(1) + CO2(g) --> (A) Ca2*(aq) + 2CI^(aq) CaCl2(aq) --> (B) CO32-(aq) + 2H*(aq) --> H20(1) + CO2(g) (C) CaCO3(s) + 2H*(aq) + 2CI¯(aq) CaCl2(aq) + H20(1) + CO2(g) --> (D) CaCO3(s) + 2H*(aq) --> Ca2*(aq) + H20(1) + CO2(g)arrow_forwardX bMy Questions | bartleby 101 Chem 101 X X app.101edu.co Submit Question 38 of 43 Write the balanced COMPLETE ionic equation for the reaction when Na2CO3 and AgNOs are mixed in aqueous solution. If no reaction occurs, write only NR. 4- |2- |2+ 3+ 4+ + 83 1 2 3 4 5 6 7 0 4 3 17 1 5 (g) (aq) (s) (1) NR Ag С N Na Reset Delete x H20 8:47 AM е OTYPE here to search 11/8/2019 S LOarrow_forward
- Balance the following reaction. If a reactant or product is not present, put a zero (0) in the blank. H+(aq) + H2O(l) + ClO-13(aq) + e-1 <--> Cl-1(g) + H+(aq) + H2O(l)arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardFor the reaction Ba(OH)2 (aq) + (NH4)½CO3 (aq)--> What one of the following species would appear in the molecular equation? (Don't worry about coefficients. Just write the molecular equation, and see which of these appears in it.) OH (s) Ba2 (aq) O NH,OH (s) BaCO3 (s) O NH2"(aq)arrow_forward
- - Part C Write a balanced ionic equation for this acid-base reaction: Ca(OH)2 (aq) + 2CH3CO₂H(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. ΑΣΦ A chemical reaction does not occur for this question. Submit Request Answer Part D ? Write net ionic equation for this acid-base reaction: Ca(OH)2 (aq) + 2CH3CO₂H(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forwardCa(ClO3)2 (aq) + NH4F (aq) -> CaF2 (s) + ? A.) Determine the product. B.) Balance the equation C.) Write the net ionic reaction D.) Identify the spectator ionsarrow_forwardbo For the chemical reaction O Macmillan Learning 2 HBr(aq) + Ba(OH)₂ (aq) → 2H₂O(1) + BaBr₂(aq) write the net ionic equation, including the phases. net ionic equation:arrow_forward
- Predicting the reactants of a neutralization reaction Predict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. → KBr(aq) + H₂O(1) × ロ→ロ Ś ? olo 18 Ar AR OF efloorarrow_forwardO Microsoft O shift V A chemistry student weighs out 0.0608 g of formic acid (HCHO₂) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1400M NaOH solution. OCHEMICAL REACTIONS Determining the volume of base needed to titrate a given mass... Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Be sure your answer has the correct number of significant digits. W Microsoft Microsoft 6.52.210... tab esc caps lock mL control Explanation ! 1 (8) Q A Check N © 2 W S #3 X T option command E x10 X D NOV 14 $ 4 C R S F do L % 5 V T G tv ♫ 6 MacBook Pro 9 Y & 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility U 8 1 9 BN M A O J K O O JOD 05 L H W P > { I command option DOCX + 11 ? | 1 9 OCarrow_forwardUse the solubility rules from your textbook to answer the following questions regarding mixtures of unknowns. If one of your test tubes contained both Ca2+andAg+, would you be able to somehow separate them by adding NaCl(aq)? Use equations to explain your answer. What if the solution contained Na+and K+? Would the addition of NaOH help you separate them?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY