
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
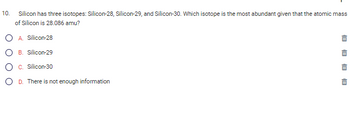
Transcribed Image Text:10.
Silicon has three isotopes: Silicon-28, Silicon-29, and Silicon-30. Which isotope is the most abundant given that the atomic mass
of Silicon is 28.086 amu?
A. Silicon-28
B. Silicon-29
C. Silicon-30
D. There is not enough information
18
18
18
18
Expert Solution
arrow_forward
Step 1
•So basically this question is based on mass of elements calculated from their isotopes and their abundance.
•So we use this formula for calculating average Atomic mass of silicon:-
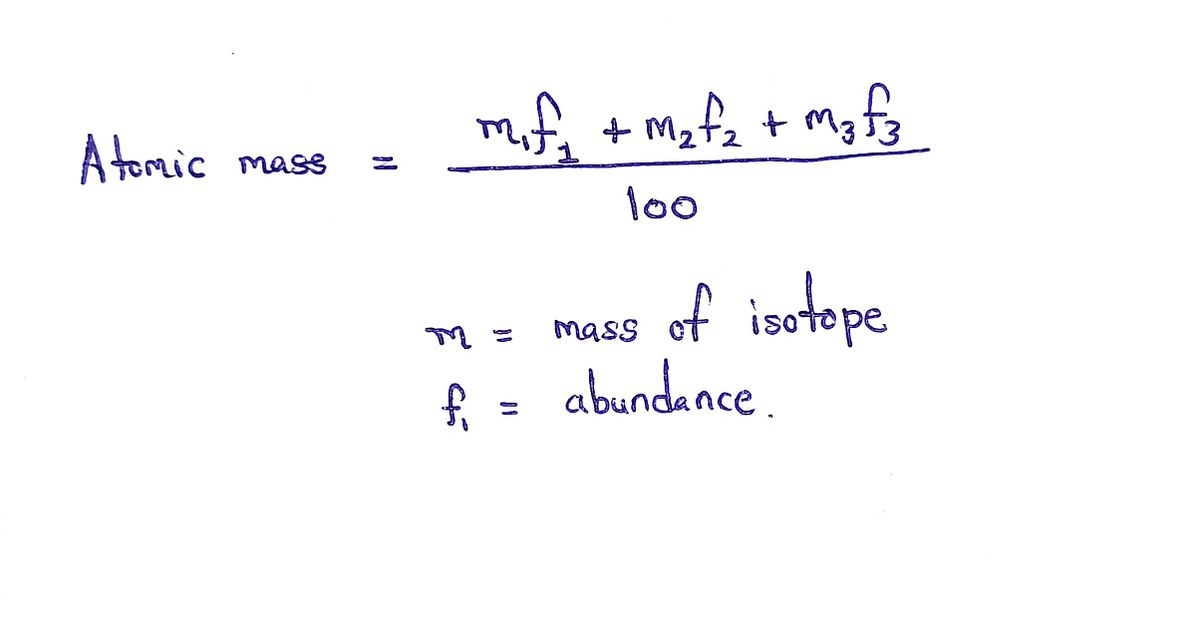
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You have a 1kg mass of each of elements A and B. Element A has a higher atomic mass than element B. Which of these is true? a. None of the others. b. A has more atoms than B. c. They have the same number of atoms, d. You cannot say with the information provided. e. B has more atoms vthan A.arrow_forward3. Carbon-14 is an isotope: A. it has the same number of protons and electrons as Carbon-12, but a different number of neutrons B. It has the same number of protons and neutrons as Carbon-12, but a different number of electrons. C. it has a different number of protons thanCarbon-12 D. it is not an isotope of Carbon-12 4. The atomic number is equivalent to which of the following? A. The number of nucleons in the atom. B. The number of protons in the atom. C. The number of neutrons in the atom. D. None of the above.arrow_forward1. Use the periodic table in the lecture to fill in the missing information regarding carbon in the table below: 6. Carbon 12.011 Element Atomic number Mass number # of protons # of electrons # of neutrons Carbon a 梦 0 hp Architectuarrow_forward
- Which of the following is not found in the nucleus of an atom? a. Electron b. Neutron c. Proton d. All of the above are found in the nucleusarrow_forwardAtomic o Mass Number of Name uon lo Symbol Number Neutrons W. Number 8. 2raon ls. 1sd: bozogorq 10 6 ros 20arrow_forward3. State the number of neutrons and protons in each of the following nuclei: a. ¹₁ H 1 179 b. C. d. 14 AU 79 6 C 239 992 Uarrow_forward
- What is the atomic mass of Cadmium ? A) 12.011 amu B) 40.078 amu C) 132.91 amu D) 112.42 amuarrow_forwardGive the number of protons, electrons, and neutrons in the following isotope: 125/52 Te Select one: a. 31 protons, 31 electrons, 52 neutrons b. 41 protons, 41 electrons, 62 neutrons c. 52 protons, 62 electrons, 125 neutrons d. 52 protons, 52 electrons, 73 neutronsarrow_forwardMCQ 32: The atom of aluminum (Al) has the number of neutrons (n) А. 13 В. 27 C. 14 D. 13arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY