
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:signment Score:
LGive Up?
O Hint
50.1%
Resources
Check Answe
estion 27 of 28>
Attempt 2
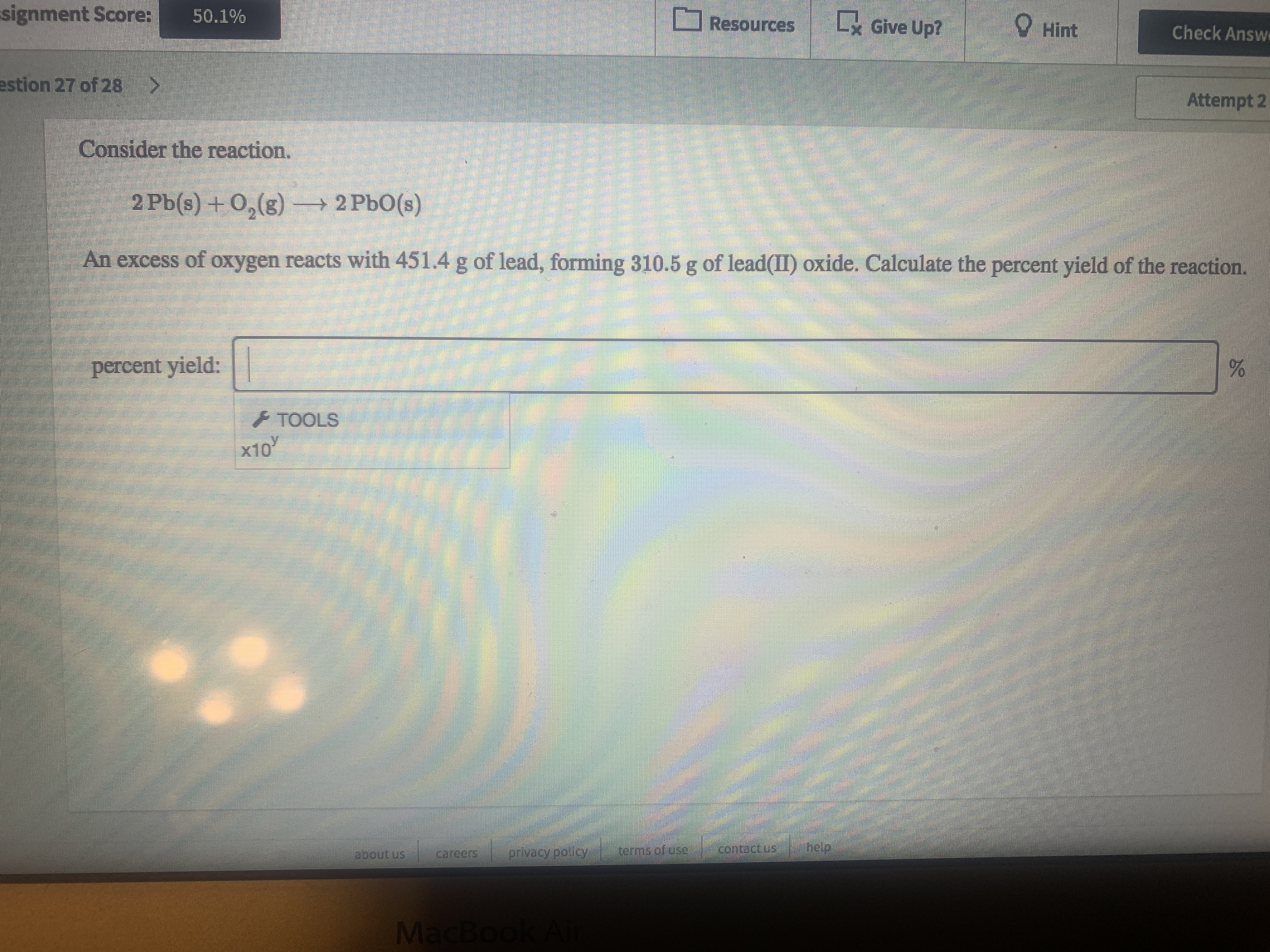
Consider the reaction.
2 Pb(s) +0,(g) – 2 PbO(s)
An excess of oxygen reacts with 451.4 g of lead, forming 310.5 g of lead(II) oxide. Calculate the percent yield of the reaction.
percent yield:
+ TOOLS
х10
about us
careers
privacy policy
terms of use,
contactus
help.
MacBo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Percent yield. 4Fe + 3O2 ---> 2Fe2 O3 Starting with 320g of oxygen gas you produce 75g of Iron(iii) oxide. What is the percent yield?arrow_forwardWindow Help ered: eq req Ereg Important values if needed... | b... M M 2req 2req 2req =2req M Visited MAY 11 S prod01-cnow-owl.cengagenow.com Submit Answer 85 According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 32.0 grams of carbon monoxide with excess oxygen gas? 2CO(g) + O2(g) → 2CO2(g) mol carbon dioxide Retry Entire Group A 6 [Review Topics] [References] Use the References to access important values if needed for this question. Module 2 | OWLv2 MacBook Pro & 87 9 more group attempts remaining * CO € 8 A S OWLV2 | Online teaching and learning resource from C... N Previous Email Instructor Next Thu May 11 10:3 zoom □ + 0 Save and Exit + 11 bl 2 201 ngsarrow_forwardQuestion 86 of 97 According to the balanced reaction below, calculate the moles of NH; gas that form when 4.2 mol of N2H. liquid completely reacts: 3 N2H.(0)→ 4 NH3(g) + N2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 4.2 28.02 5.6 17.04 16.8 3.2 32.06 6.022 x 10 mol NH3 mol N2H4 g N2 g NH3 g N2H4 mol N2 MacBook Airarrow_forward
- Qlaccd sign in - Search C tab Ug ift T O CHEMICAL REACTIONS Theoretical yield of chemical reactions esc caps lock Explanation K →1 Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H₂O). What is the theoretical yield of sodium bromide formed from the reaction of 12.9 g of hydrobromic acid and 11.7 g of sodium hydroxide? Be sure your answer has the correct number of significant digits in it. LION E fn https://www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym 11 X ALEKS Type here to search ? A Check 2 Z W S # * 3 X alt E D 14 $ x10 X с 7. 4 S f5 % 40 5 X At V C. T Me Graw F McGraw-Hill Education Campus X A ALEKS-Shushanik Babayan - Le X + 96sW-8PcvpF38ZsC... Al 4- G f7 4+ & Y H NO © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Access a ? f8 IAA * B N J fg DII M K f10 F11 0 f12. alt 3/5 insert 94°F [ C. prt sc ] Sh pause ctrl 6 7/1 backsarrow_forwardstion O Macmillan Learning Combining 0.384 mol Fe₂O3 with excess carbon produced 16.5 g Fe. Fe₂O3 + 3C 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: What is the theoretical yield of iron in moles? theoretical yield: What is the percent yield? percent yield: Search or type URL 1 . 6 MacBook Pro & r 7 * 8 + ( 9 ) 0 w + II mol mol %arrow_forwardHow many moles of H2SO4 are required to completely react with 7.20 mol of Al according to the balanced chemical reaction: 2 Al(s) + 3 H:SO-(aq) → Al:(SO-):(aq) + 3 H2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 21.6 26.98 6.022 x 1023 2 7.20 2.40 3.60 1 98.08 342.14 3 2.02 10.8 g Al mol Al:(SO.)s g Al:(SO4)s mol Hz mol Al g H2SO. mol H2SO4 g H2 Iarrow_forward
- Pls help ASAP.arrow_forward2 NAHCO:(s) > I Na,0(s) 2 co.(e) H;O(8) Reaction B 4.476 Run 1: Initial grams of NaHCO3 Theoretical yield (grams) of Na,0: Percent yield (Run 1) =arrow_forwardExplosive exnon trioxide, XeO3, is prepared as in the below unbalanced equation. In one expriment, 3.57 g of xenon trioxide was collected after reacting 8.62 g of XeF6 with an excess of water. What is the percent yield? XeF6 + H2O ------> XeO3 + HF (unbalanced)arrow_forward
- 40 1:28 2. 2 Cla(g) + 3 Al(s) - 2 AICla(s) 1 a) Calculate the theoretical yield of aluminum chloride (in grams) that can be produced from 13 grams of aluminum metal. b) A student performed this experiment and obtained 29.5 grams of aluminum chloride. Determine the percent yield of aluminum chloride. 65/96arrow_forwardAssignment Score: 13.5% Give Up I Resources O Hint Check Answer Question 23 of 26 > Combining 0.220 mol Fe,0, with excess carbon produced 18.6 g Fe. Fe,0, +3C - 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: mol What is the percent yield? percent yield: cerarrow_forwardA Login My AP Login - Coll. LanguageTool -Onl.. Co Biography of Albert. Crillegnettouth Pre-AP Unit 3 Learning Checkpolnt 2 1 (3 10 11 Question 9 D CH, (g) + 2 O2 (g) CO, (g) + 2 H20 (g) When CH, (g) is burned in O,(g), the reaction represented by the equation occurs. If 32 g of CH, is burned completely, how many moles of CO, are produced? Enter the number of moles to the nearest whole number. molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY