
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
-
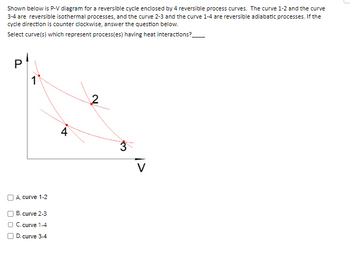
Shown below is P-V diagram for a reversible cycle enclosed by 4 reversible process curves. The curve 1-2 and the curve 3-4 are reversible isothermal processes, and the curve 2-3 and the curve 1-4 are reversible adiabatic processes. If the cycle direction is counter clockwise, answer the question below.
Select curve(s) which represent process(es) having heat interactions?____
A. curve 1-2
B. curve 2-3
C. curve 1-4
D. curve 3-4
Transcribed Image Text:Shown below is P-V diagram for a reversible cycle enclosed by 4 reversible process curves. The curve 1-2 and the curve
3-4 are reversible isothermal processes, and the curve 2-3 and the curve 1-4 are reversible adiabatic processes. If the
cycle direction is counter clockwise, answer the question below.
Select curve(s) which represent process(es) having heat interactions?
P
1
A. curve 1-2
B.
C. curve 1-4
D. curve 3-4
curve 2-3
4
2
3
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Thermodynamics fill in the blank: 1) Among the principal irreversiblilities of a gas turbine power plant, the most significant source of irreversibility by far is ______________. 2) Cooling a gas as it is compressed would reduce the work input required during compression, but achieving a heat transfer rate high enough to effect a significant reduction in work is difficult to achieve in practice. A practical alternative is to introduce multiple stages of compression with heat exchangers called _______________ between the stages.arrow_forwardA 2.00 mol sample of an ideal diatomic gas is taken through a reversible cycle A → B → C → A as shown in the figure below. Process A → B is isothermal expansion. For the cycle, calculate: a) the work done by the gas, b) the heat energy added to the gas, c) the heat energy exhausted by the gas, d) the thermal efficiency, e) the change in entropy of the gas. f) Determine the efficiency of a Carnot engine operating between the same temperature extremes, R = 8.314 J/mol K Data at vertices for the above PV diagram are as follow: PA = 5.00 x 105 Pa, VA = 1.00 x 10 -2 m3, PB = 1.25 x 105 Pa, VB = 4.00 x 10 -2 m3 PC = 1.25 x 105 Pa, VC = 1.00 x 10 -2 m3arrow_forwardExplain the heat transferred from a furnace (Q) to convert the boiler feed water at 25 ° C into superheated steam at 17 bar and 250 ° C.arrow_forward
- 4. What is the second law of thermodynamics, give its Clausius and Kelvin-Planck statements and justify on their basis why a perpetual motion machines that extracts heat from ambient environment to do work is not feasible. What is difference between reversible and an internally reversible process, and why are most actual processes considered irreversible. Numerical:arrow_forwardThermodynamicsarrow_forwardstream? Q2/ An ideal gas initially at 600 K and 10 bar undergoes a four step mechanically reversible cycle in a closed system. In step 1-2, pressure decreases isothermally to 3 bar; in step 2-3, pressure decreases at constant volume to 2 bar; in step 3-4, volume decreases at constant pressure; and in step 4-1, the gas returns adiabatically to its initial state. Assum C₂=(7/2) R and C₂ = (5/2) R. Calculate Q, W, AU, and 4H for each step of the cycle?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY