Question
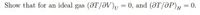
Transcribed Image Text:Show that for an ideal gas (@T/ƏV)u = 0, and (@T/ƏP)H= 0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- the partition function of an ideal gas of diatomic molecules in an external electric field & is [g(V, T, 8)]" Q(N, V, T, 8) N! where (2mmkT 312 (87 IkT -hv/2kT e q(V,T, 8)= V{ h2 (kT' (µ8 sinh kT) h2 (1 – e-hv/kT) Here I is the moment of inertia of the molecule; v is its fundamental vibrational frequency; and u is its dipole moment. Using this partition function along with the thermodynamic relation, dA = -S dT –p dV – M de where M=Nū, where u is the average dipole moment of a molecule in the direction of the external field &, show that kT] coth kT, Sketch this result versus & from & =0 to & =∞ and interpret it.arrow_forwardWhat is the root-mean-square speed, in units of m/sec, of monatomic Ne atoms at a temperature of 24.1°C?arrow_forwardhi, please I need answer for a,b and c.arrow_forward