
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
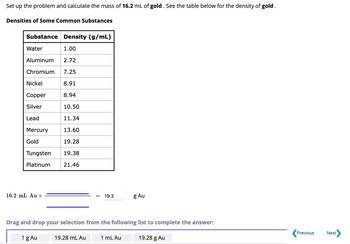
Transcribed Image Text:Set
up the problem and calculate the mass of 16.2 mL of gold. See the table below for the density of gold.
Densities of Some Common Substances
Substance Density (g/mL)
Water
1.00
Aluminum 2.72
Chromium 7.25
8.91
8.94
10.50
11.34
13.60
19.28
19.38
21.46
Nickel
Copper
Silver
Lead
Mercury
Gold
Tungsten
Platinum
16.2 mL Au X
1 g Au
19.3
Drag and drop your selection from the following list to complete the answer:
19.28 mL Au
g Au
1 mL Au
19.28 g Au
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Density of a Solid (Aluminum) Run I Run II Mass of solid (M) 41.386 g 35.387 g Volume of water 25.32 mL 25.14 mL Volume of water + solid 40.65 mL 38.86 mL Volume of solid (V) mL mL Density of solid (D = M/V) g/mL g/mL Average density g/mL Accepted value for density of solid g/mLarrow_forward0.161 STARTING AMOUNT 0.161 km km + 10€ SHSSFE生 0.1 A city block is 0.161 km long. How many cm is this? 0.0161 0.000161 16100 161 0.001 0.00161 km 10 m 0.01 1 cm 1610 10€ 0.161 1000 100 16.1arrow_forwardA firkin = 40.82 kg (inexact). How many firkin in 76.2 kg? 3110 fir O 1.866 fir 1.87 fir 3.21x10-4 fir 0.5357 firarrow_forward
- The mass of a certain atom is 2.086 x 10°22 g. This is the same mass as 2.086 x 10-16 mg. 2.086 x 10-25 kg. 2.086 x 10-28 Hg. O 2.086 x 10-31 ng.arrow_forwardA 0.235 mL sample of carbon tetrachloride (density = 1.59 g/mL, MM = 153.81 g/mol) contains how many AMU of Cl? Note: Use 6.022 x 1023 and/or 1.661 x 10-24 as appropriate. 5.18 x 1022 2.42 x 10-3 2.07 x 1020 1.30 x 1022 2.07 x 1023 0.333 g of a hydrocarbon (CxHy) was analyzed by combustion analysis and 1.009 g of CO2 (MM = 44.01 g/mol) and 0.516 g of H2O (MM = 18.02 g/mol). Which of the following is the correct empirical formula of the hydrocarbon? C2H4 C2H5 C3H7 C2H3 CH3arrow_forwardNeed to verify the significant figures in these mass measurements and subsequent calculations using those measurementsarrow_forward
- Convert the following chemicals in your recipe to their respective mass in grams and then to a standard cooking measurement. Baking Soda Mass Standard Cooking Measure (1 tsp = 4.87 g) All-Purpose Flour (Use C4H8O4 as the chemical formula) Mass Standard Cooking Measure (1 cup = 132.09 g)arrow_forwardThere are more answers than what is shown in the image.arrow_forwardSet up the problem and calculate the volume occupied by 17.4 grams of gold. See the table below for the density of gold. Densities of Some Common Substances Substance Density (g/mL) Water Aluminum Chromium Nickel Copper Silver Lead Mercury Gold Tungsten Platinum 17.4 g Au x 1.00 2.72 7.25 8.91 8.94 10.50 11.34 13.60 19.28 19.38 21.46 mL Au Drag and drop your selection from the following list to complete the answer: 1 mL Au 19.28 g Au 19.28 mL Au 1 g Au Previous Nextarrow_forward
- Set up the problem and calculate the mass of 16.6 mL of gold. See the table below for the density of gold. Densities of Some Common Substances Substance Water 1.00 Aluminum 2.72 Chromium 7.25 8.91 8.94 10.50 11.34 13.60 19.28 19.38 21.46 Nickel Copper Silver Lead Mercury Gold Density (g/mL) Tungsten Platinumarrow_forwardMultiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 93.4 0.934 mol L Explanation 988.9 mol + 0.68 L = [ mol L X 26. L = = mol Check × 1.35 L = mol W mol 3 E 65 X MacBook Pro 7 2022 McGraw Hill LLC. All Rights Reserved. Term=arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY