
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
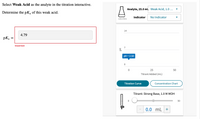
Transcribed Image Text:Select Weak Acid as the analyte in the titration interactive.
Analyte, 25.0 ml. Weak Acid, 1.0 ...
Determine the pK, of this weak acid.
Indicator
No Indicator
colorless
14
4.79
pKa
Incorrect
pH = 2.50
25
50
Titrant Added (mL)
Titration Curve
Concentration Chart
Titrant: Strong Base, 1.0 M MOH
50
0.0 mL +
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 25.00 mL of a weak monoprotic acid is titrated with a 2.00 M solution of NaOH. The following data is collected mL NaOH added PH 5.55 half-way point 2.50 equivalence point 5.00 saved Use the pH at the half-way point point to estimate the pK, of the acid. 00000 5.55 6.43 05.12 8.76 6.01 O 5.92arrow_forwardA Consider the pH titration curve above (pH vs volume in ml). The pk, of the weak base being titrated and the pH of the equivalence point are, respectively: 12 11 10 8 7 5 4 2 1 10 20 30 40 O 2.5 and 4.0 • 5.4 and 4.5 O 11.2 and 5.4 O 11.2 and 4.5 O 4.5 and 5.4 Based on the indicator information above, which of the following indicators could be used successfuly in the titration in Part A? (Select all correct responses. Indicator Colour (low pH – high pH) pK, methyl yellow red-yellow 3.1 methyl orange red-yellow 3.7 bromophenol blue yellow-blue 4.2 bromocresol green yellow-blue 4.7 methyl red pink-yellow 5.1 bromocresol purple bromothymol blue yellow-purple 6.3 yellow-blue 7.0 phenol red yellow-red 7.9 O phenol red cresol red yellow-red 8.3 O bromocresol purple thymol blue yellow-blue 8.9 O methyl yellow thymolphthalein colourless-blue 9.2 O bromothymol blue O thymol blue O thymolphthalein Part C Baseu on the table above, what colour is methyl orange at pH3? Submit Request Answer Part…arrow_forwardI really need help with this review problem thanksarrow_forward
- Determination of K, TRIAL Volume NaOH at pH at Midpoint of Titration Midpoint of Titration 1. 3.22 3. Average K Identity of Unknown Acidarrow_forwardA 24.0 mL sample of 0.115 M sulfurous acid (H,S03) is titrated with 0.1019 M KOH At what added volume of base solution does the first equivalence point occur? Express your answer in mililiters to three significant figures. V. mL Submit Request Answer Vorue nertlelarrow_forwardLearning Goal: To calculate the pH at the equivalence point for various types of titrations. The equivalence point in an acid-base titration is the point at which stoichiometrically equivalent quantities of acid and base have been mixed together. At this point the reaction is complete because all analyte has been consumed by titrant. On a titration curve, the equivalence point is represented by the point of inflection (where the curve changes concavity). The figure (Figure 1) shows the titration of 40.0 mL of 0.100 M HCl with 0.100 M NaOH. When 40.0 mL of the NaOH solution is added, the acid-base neutralization reaction is complete. When analyzing titrations involving weak acids or bases, consider how the neutralization reaction will impact the pH of the system. For example, when titrating a weak acid with a strong base, at the equivalence point all the base has been used to neutralize the acid forming its conjugate weak base Figure E 14 12 10 8 6 4 2 0 0 Equivalence point 20.0 40.0 mL…arrow_forward
- Find moles of NaOH added and equivalence pointarrow_forwardA 100.0 mL acetic acid buffer solution contains 0.50 M CH3COOH and 0.50 M CH3COONa. What will the pH be after 0.020 mol of HCI has been added to the buffer solution? pk, of acetic acid = 4.74 5.11 4.74 8.89 None of these 9.62 4.38arrow_forwardM of NaOH: 0.100 Volume of HCI 25mL = 0.025L Moles of HCI = 2.5 mol What is the Volume of NaOH equivalence point: What is Moles of NaOH equaivalence point: What is pH at equivalence point? What is calculated pH and experimental pH of NaOH at 0.00 mL , 12.50 mL, 25mL, and 30 mL. Also what is the Error percentage for each one?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY