
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
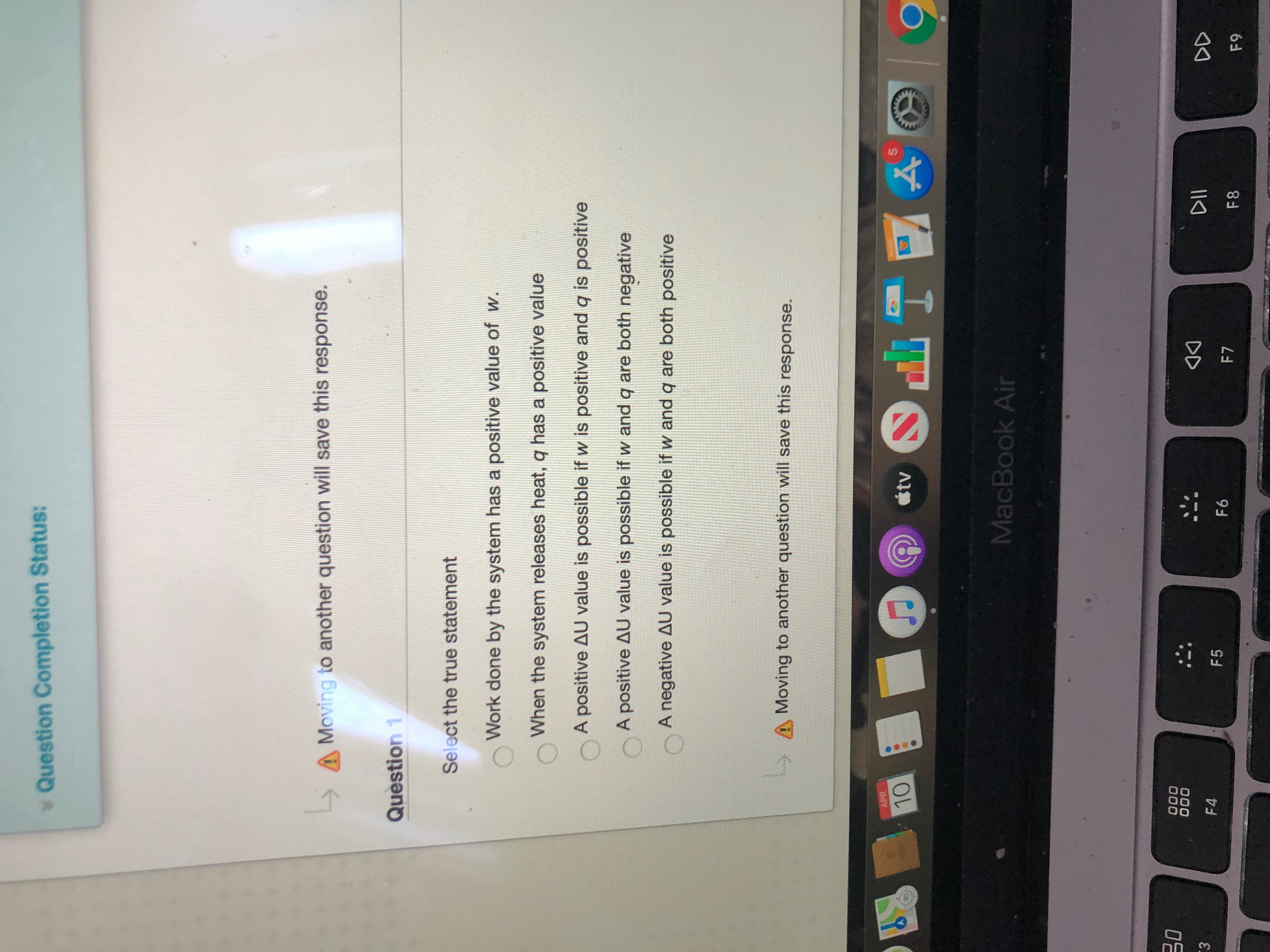
Transcribed Image Text:Select the true statement
Work done by the system has a positive value of w.
When the system releases heat, q has a positive value
A positive AU value is possible if w is positive and q is positive
A positive AU value is possible if w and q are both negative
A negative AU value is possible if w and q are both positive
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. Calculate the specific heat of a piece of wood if 1500.0 g of the wood absorbs 65,000 joules of heat, and its temperature changes from 32°C to 57°C. SHOW YOUR WORK Variables Calculate here: m = C = AT =arrow_forwardCan some show me how to calculate the specific heat with the given information?arrow_forwardCalculate the work done when a gas contracts from a volume of 22.00 L to a volume of 10.00 L against a constant external pressure of 1.50 atm. -2.28 kJ O 4.10 kJ O-3.04 kJ O 1.82 kJ O-5.93 kJarrow_forward
- A chemical reaction is run in which 308 Joules of work is done on the system and the internal energy changes by + 84 Joules.Calculate q for the system.q =________ Joulesarrow_forwardA cylinder and piston system releases 328 J of heat and does 144 J of work pushing the piston against external pressure. State the value of q for this process with the correct sign. J State the value of w for this process with the correct sign. J State the value of the change in internal energy, AE, for this process with the correct sign. Jarrow_forwardThe question is given like this, it is not incomplete. There is no heat capacity of the calorimeter given. Can it be solved without that? It is high school chemistry so maybe it can be solved in an easier way? Many thanks.arrow_forward
- Calculate the change internal energy (AE or AU) for a system that is gaining 65.0 kJ of heat and has 855 J of work performed on it by the surroundings. Be mindful of units and signs. 65.9 kJ O -64.1 kJ O -65.9 kJ -65.0 kJ O 64.1 kJarrow_forwardA cup of water (the system) is heated in a microwave oven until it begins to visibly boil. State whether each of q, w, and ΔU is positive, negative, or zero.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY