
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:KINETIC ENERGY (3)
10
9
T
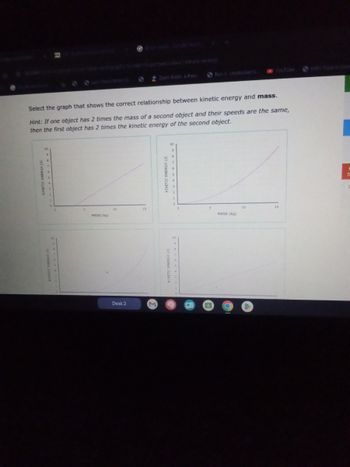
Select the graph that shows the correct relationship between kinetic energy and mass.
Hint: If one object has 2 times the mass of a second object and their speeds are the same,
then the first object has 2 times the kinetic energy of the second object.
KINETIC ENERGY (1)
g****
MASS (kg)
10
S 2 Zeam Math: A Pers
Desk 2
15
M
KINETIC ENERGY (J)
KINETIC ENERGY (3)
10
10
Run 3-Unblocked G
E
MASS (kg)
YouTube
10
15
I
:
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Peanut Marshmallow Mass of Water 50g 50g Initial Mass of Food Sample and Paper Clip 10.42 g 14.44 g Change in Water Temperature (°C) 58.5 20.7 Final Mass of Food Sample and Paper Clip After Burning 9.02 g 13.68 g Determine the number of kilocalories (food Calories) released by the burning food sample (1 kilocalorie, or Calorie = 1,000 calories). Q = ____________ kilocalories (food Calories) peanut Q = ____________ kilocalories (food Calories) marshmallowarrow_forwardCalorimetry Chart Mass of Food 20.7 g 3.2 g Mass of Food residue (after burning) Volume of water heated 94.2 ml Initial Temperature of water Final Temperature of water 13.6°C 78.3°C How many Calories are in the food in the data table? * O 1.13 Calories O 6.09 Calories O 1.37 Calories O 7.38 Calories DELLarrow_forwardA piece of brass with mass of 1750 g is heated. The brass is dropped into a 250.0g aluminum calorimeter cup containing 600.0g of water. The temperature of the water goes from 15 degrees celsius to 80 degrees celcius. What is the initial temperature of the brass?arrow_forward
- Pls help ASAP. Pls give proper units and significant digits to three. Pls circle the final answer as well.arrow_forwardi need help answering this question pleasearrow_forwardWhich of the following types of energy is/are classified as potential energy? 1.Thermal energy 2.Energy stored in a spring 3.Gravitational energy (A) 2 and 3 1 only 2 only 3 only 1, 2, and 3arrow_forward
- For the following questions (Q11-Q14), consider the compound NH4Cl, ammonium chloride, having a molar mass of 53.491 g/mol. Nicole weighs out 12.41g of NH4Cl and also 125.0 g of water for her calorimetry experiment. Nicole dissolves her ammonium chloride in the water and finds that the temperature of the water decreases from 23.7°C to 17.3°C. Assume the specific heat capacity of the water, Cs,water, is 4.184 J/(g °C) and that no energy is transferred to the calorimeter (qcal = 0 J) Q11. Find the heat (in J) transferred to the water, qwater, during the process NH4Cl (s) ® NH4Cl (aq). Use the equation qwater = mwater Cs,water ∆T. Q13. Using your result in Q11, calculate the enthalpy of reaction, ∆Hrxn, in units of kJ/mol.arrow_forwardWhat is the kinetic energy of a 23.1 g ball moving at 39.1 m/s? Report your answer with the correct number of significant figures. Do not include units in your answer.arrow_forwardConvert the heat of neutralization of acetic acid from -48.6 kj/mmol to calories per millimole and ROUND TO THE NEAREST WHOLE NUMBER AND INCLUDE THE APPROPRIATE SIGN (1 cal = 4.184 J) DO NOT INCLUDE UNITS Type your answer...arrow_forward
- question is in the photoarrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 165.0 g sample of a pure substance from -8.5 °C to 15.1 °C. The experiment shows that 541. J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits. 1 J.g .K - 1 x10 X 3arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 1.57 kg sample of a pure substance from-1.1 °C to 8.2 °C. The experiment shows that 6.6 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits. -1 DJ-g K -1 10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY