
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
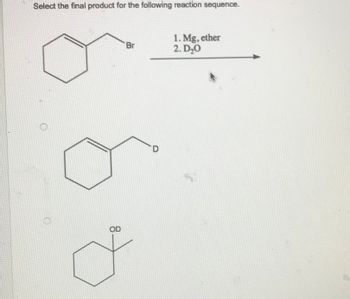
Transcribed Image Text:Select the final product for the following reaction sequence.
OD
Br
D
1. Mg, ether
2. D₂0
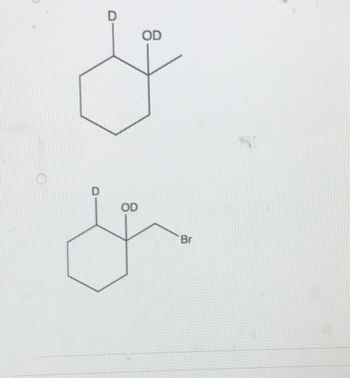
Transcribed Image Text:D
OD
D
OD
&
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name if the reaction for the synthesis of Benzhydrol? What is the full reaction mechanism? Benzhydrol MgBr 1. ether 2. H*, H₂O OHarrow_forwardGive the major organic product for each step of the following reactionarrow_forward6. What is the major organic product obtained from the following reaction? Brzarrow_forward
- 11. Draw the structure for the molecule that would yield the products shown below under the indicated reaction conditions. Br 1 molar eq HBr -78 °C 1 molar eq HBr 50 °C Brarrow_forwardWhat are the major products of these reactions?arrow_forwardGive starting materials, reagents, and conditions that will produce each product.arrow_forward
- Predict each product for the following multistep reaction. If ortho/para products are both formed, use the para product in the next reaction. 46. Predict each product for the following multistep reaction. If ortho/para products are both formed, use the para product in the next reaction. Br2 FeBr3 HNO3 A H2SO4 B Fe C HCI NaNO3 CuCN D E H₂O F Garrow_forward7. The reaction of methoxide anion with bromoethane to yield the ether ethyl methyl ether and the bromide anon (Br-) is an excellent example of a general reaction type called Sy2 (substitution nucleophilic bimolecular): CH,0+ CH,СH-Br a CH3-0-CH,СH; + Br- a. Change in enthalpy is -103 kJ/mol; the change in entropy is + 0.025 kJ/mol-K. Calculate DG at 300K. b. Is the reaction endergonic or exergonic? c. Is the reaction endothermic or exothermic? d. Use curved arrows to show the complete mechanism. Reaction of 2-methyl-1-butene with H-Cl could yield TWO alkyl chloride products. Draw and name 8. them.arrow_forward12. Draw the structure of the major organic product(s) for the following reactions and name the reaction. CH3 N-CH3 CI CH3 heat a. (name of reaction) 0- N-CH3 CI CH3 b. (name of reaction) -NH2 + CH3CH2Cl c. (name of reaction) heat NaOH 2 H d. name of reaction H3C e. name of reaction CH3 1. EtQ Na+ 2. H2O, HCIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY