
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
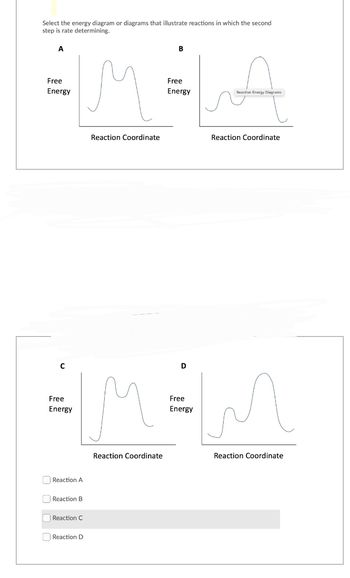
Transcribed Image Text:Select the energy diagram or diagrams that illustrate reactions in which the second
step is rate determining.
A
Free
Energy
C
Free
Energy
Reaction A
Reaction B
Reaction C
Reaction D
Reaction Coordinate
B
Reaction Coordinate
Free
Energy
Reaction Energy Diagrams
Reaction Coordinate
D
Free
M=MM
Energy
Reaction Coordinate

Transcribed Image Text:Select all of the following that would occur to an enzyme-catalyzed reaction in
the presence of a reversible, uncompetitive enzyme inhibitor (compared to the
same reaction in the absence of the inhibitor).
Vmax would increase
Km would increase
Vmax would decrease
Vmax would not change
Km would decrease
Km would not change
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following energy diagrams is a reaction with one intermediate? ... reaction coordinate ... reaction coordinate free energy, kJ/mol free energy, kJ/molarrow_forwardThe question is attachedarrow_forwardDraw the arrows showing how electron move to get from reactant to product ?arrow_forward
- Considering each of the following values and neglecting entropy, tell whether the starting material or product is favored at equilibrium: (a) ?H° = 80 kJ/mol; (b) ?H° = -40 kJ/mol.arrow_forwardCompound A is heated in methanol B to give product C. a. Draw product C and provide a detailed mechanism for its formation. OSO₂CH3 + CH3OH A A B Carrow_forwardConsider the reaction coordinate diagram shown. Which step has the greatest rate constant going in the forward direction? Free Energy O O A E going to F OC going to E E going to G OC going to A A going to C C D Progress of rxn E Farrow_forward
- 1. For a reversible reaction at an equilibrium: A. The rate of the reaction forward is the same as that of the reaction in reverse. B. The rate constant for the reaction forward is the same as that for the reverse. C. The free energy of substrate is the same as that of product. D. No conversion of substrate to product, and product to substrate occurs. E. Position of an equilibrium does not depend on substrate and product concentration, only on the magnitude of equilibrium constant.arrow_forwardWhat is the effect of halving the amount of ethanol for this reaction? HO, Br heat A. the reaction rate decreases by half B. C. D. the reaction rate stays the same the reaction rate doubles the reaction rate quadruplesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY