
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
#20
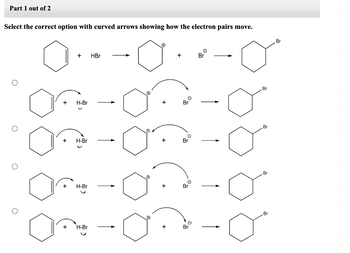
Transcribed Image Text:Part 1 out of 2
Select the correct option with curved arrows showing how the electron pairs move.
+ HBr
+ H-Br
+ H-Br
J
On
H-Br
H-Br
Ⓒ
O
+
+
+
O
Br
Br
O
Br
Br
e
Br
Br
Br
Br
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A cake requires 3 eggs, 4 cups of flour, 2 cups of sugar, 0.5 cups of oil, and 1 stick of butter. In the refrigerated pantry, a chef has the following available: 56 sticks of butter 60 eggs 12 gallons (192 cups) of oil 56 cups of flour 41 cups of sugar Select the four excess reagents. Do not select the limiting reagent. butter flour eggs sugar oilarrow_forward1arrow_forwardCan you explain?arrow_forward
- Write the IUPAC name for each of the following:arrow_forwardQUESTION 19 On the phase diagram below the triple point is_(1), the critical point is _(2), and the line representing melting is_(3)_. 4.0 E P (atm) 3.0 2.0 1.0 30 A F 40 ‚B → H G C 50 60 70 T (degrees C) D 80arrow_forwardHow many secondary hydrogens does the following compound have? Select one: OA. 10 OB. 6 OC. 4 D. 12 8 P Flag question OE 4 Xarrow_forward
- NEITEHR OF THE ANSWERS SHOULD BE PSI -60 PLEASE IF YOU PUT THAT I WILL DISLIKE AND If you copy ur answers from other bartleby answers i will dislikearrow_forward1. Fill in the box with the missing starting material(s), reagent(s), or product(s): 1.1 1.2 1.3 1.4 1.5 OH Br 1 eq. 1eq. HBr H3O+ X AICI 3 LDA CI H+/MeOH CF3 НА омоarrow_forward#15arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY