
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
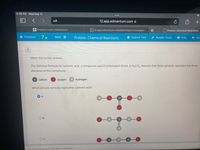
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Be sure to answer all parts. Find the pH and the volume (mL) of 0.347 M HNO3 needed to reach the equivalence point in the titration of 2.65 L of 0.0750 M pyridine (C5HƑN, K₂ = 1.7 × 10¯). Volume = mL HNO3 PH =arrow_forwardTrue or Falsearrow_forwardPart A For the reaction Br2 (1) 2 Br (g) at what temperature is the equilbrium constant equal to 3.5? t> VO ΤΙ ΑΣΦ 0 ? Your answer should not contain commas. No credit lost. Try again. Submit Previous Answers Request Answer Karrow_forward
- atoms. 12 hydrogens, 6 oxuger Click or tap here to enter text.C atoms. 12) Back to the person who ate 300 grams of glucose, how many moles of glucose is this? Click or tap here to enter text. There are 2 moles. 13) If a person were to eat this much glucose all at once (that is a lot of sugar) and all this sugar enters the blood then the person's blood sugar concentration would rise. Imagine that all this glucose is released into the 5 liters of blood that circulate in this person. What would be the molarity of glucose in the blood? Click or tap here to enter text. 14) After each of these glucose molecules enter the blood they are uptaken through cell membranes to enter cells. Within these cells glucose is broken down in a series of steps releasing carbon dioxide along the way. For every molecule of glucose broken down how many molecules of carbon dioxide are created? Refer to the cellular respiration equation: Equation for Cellular Respiration: How to break down a carb C6H12O6 + 60₂ ➜…arrow_forwardCombining 0.304 mol Fe2O30.304 mol Fe2O3 with excess carbon produced 11.6 g Fe.11.6 g Fe. Fe2O3+3C⟶2Fe+3COFe2O3+3C⟶2Fe+3CO What is the actual yield of iron in moles? What is the theoretical yield of iron in moles? What is the percent yield?arrow_forwardNonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON