
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
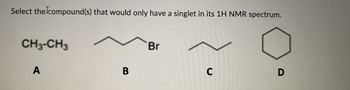
Transcribed Image Text:Select the compound(s) that would only have a singlet in its 1H NMR spectrum.
CH3-CH3
A
B
Br
C
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A different unknown has an FTIR with a very intense peak at 1735 cm. Its 13C NMR spectrum exhibits a peak at 169.3 ppm, and the 'H NMR spectrum shows a quartet at 4.07 ppm. Which of the following compounds is the unknown most likely to be? (a) (b) (c) (d) (e) O compound (a) O compound (d) compound (e) compound (c) compound (b) (0)arrow_forwardDraw a mechanism of a reversible reaction of acetophenone and strong acid (H-A) to form a protonated acetophenone and a weak base. Draw all the electron pairs of the molecules and the arrows. Predict 1H NMR spectra of acetophenone and the protonated acetophenone.arrow_forwardWhich xylene compound is the hardest to differentiate from 1,3,5-trimethylbenzene using ¹H NMR? Lá A A B C Barrow_forward
- Match each structure to the appropriate NMR by placing the letter of the compound in the box on the NMR it corresponds to. The aromatic region has been included as an inset to make splitting patterns easily visible.arrow_forwardMatch each of the following 1H NMR spectra with one of the following compoundsarrow_forward5. Which type of spectroscopy (IR, ¹H NMR, 13C NMR) would be good to distinguish between benzaldehyde and benzonitrile? State what you would observe for each compound in each type of spectroscopy and whether that type of spectroscopy would be a good way to distinguish between the two compounds. benzaldehyde IR ¹H NMR 13C NMR benzonitrile Good way to distinguish?arrow_forward
- (b) In the IR spectrum of the product, which signals are due to the carbonyl and the alkene? NMR dibenzalacetone (expanded) 2 NMR diberzalacetone lancre this peak Ignore this peak 6 Ignore this peak PPMarrow_forwardHow many signals will this compound produce in a 1H NMR spectrum?arrow_forwardA. How many signals would you expect in the 'H NMR spectrum of the following compound? B. How many signals would you expect in the 13C NMR spectrum of the following compound? C How many signals would you expect in the 13C NMR spectrum of the following compound? D₁ How many signals would you expect in the 13C NMR spectrum of the following compound? E How many signals will be expected in the ¹H NMR spectrum of the following compound? NO₂ For each of the molecular formulas shown below, there is a compound that exhibits a 'H NMR spectrum with only one signal. Draw the ■tructure in each case. C₂Ho Draw Your Solution eTextbook and Media CsHyCla CH Draw Your Solution Draw Your Solutionarrow_forward
- 3 please answer all 3 parts .a)The 1H NMR, 13C NMR, and the IR spectra of compound 2 are shown below. The molecular formula is C7H14O. What is the structure?b)The 1H NMR, 13C NMR, and the IR spectra of compound 1 are shown below. The molecular formula is C4H8O. What is the structure? c)Compound 1 and Compound 2 were formed from ozonolysis. What was the starting alkene that Professor Jackson had? The molecular formula is C11H22.arrow_forward1H NMR Analysis in the picture what is the Product Name? and please assign the shifts and peaksarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY