
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please help with missing blanks
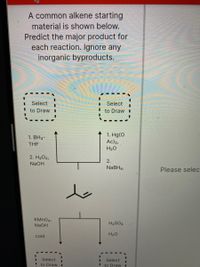
Transcribed Image Text:**Title: Alkene Reaction Pathways**
**Introduction:**
A common alkene starting material is shown below. Predict the major product for each reaction. Ignore any inorganic byproducts.
**Reaction Pathways:**
1. **Pathway 1: Hydroboration-Oxidation**
- Reagents:
1. BH₃-THF
2. H₂O₂, NaOH
- Mechanism: This pathway involves the hydroboration of the alkene followed by oxidation to form an alcohol.
2. **Pathway 2: Oxymercuration-Reduction**
- Reagents:
1. Hg(OAc)₂, H₂O
2. NaBH₄
- Mechanism: This pathway includes the oxymercuration of the alkene followed by reduction, resulting in the formation of an alcohol.
**Diagram:**
- **Alkene Structure:** The starting material is an alkene with two substituents around the double bond, depicted graphically in the center.
- **Reactions Arrows and Boxes:**
- Each reaction pathway leads to another box indicating the site for student input, labeled "Select to Draw," where learners can draw predicted products.
3. **Pathway 3: Cold Alkaline Permanganate Oxidation**
- Reagents: KMnO₄, NaOH, cold
- Mechanism: This process involves the syn-dihydroxylation of the alkene, forming adjacent diols.
4. **Pathway 4: Acid-Catalyzed Hydration**
- Reagents: H₂SO₄, H₂O
- Mechanism: An acid-catalyzed process resulting in alcohol formation via a carbocation intermediate.
Each reaction pathway guides students through understanding different reaction mechanisms leading to various functional groups. Students can apply knowledge of reagents, reaction conditions, and mechanisms to predict major products effectively.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 2arrow_forwardLevel = 6ft of water (h20 density = 0.036 Ib/in^3) Find gauge reading in psi = Find gauge reading psig = Find gauge reading in psia =arrow_forwardThe standard graph contains absorbance values greater than 1.00 Is there a positive error, negative error, no effect, or error could not be determined In terms of the Linearity of the standard curve?arrow_forward
- How can we identify whether the compounds are ionic or covalent for part 1?arrow_forward2) What is the relative error in delivering a volume of 18.28 mL from a class A 25 mL buret? (Remember, burets require two readings to deliver a certain volume, pipets only require one reading!) NIST Tolerances for Volumetric Glassware, Class Aa Tolerances (mL) Capacity (mL) (Less Than and Including) Volumetric Flasks Transfer Pipets Burets 1000 +0.30 500 ±0.15 100 +0.08 ±0.08 +0.10 50 ±0.05 +0.05 +0.05 25 +0.03 +0.03 +0.03 10 +0.02 +0.02 +0.02 5 +0.02 +0.01 +0.01 2 +0.006 "Corning Pyrex glassware and Kimball KIMAX, Class A, conform to these tolerances.arrow_forward25 Choose the letter of the correct answer.arrow_forward
- How is light and energy used in gas chromatography- mass spectrometry (GC-MS)?arrow_forwardCalculate the standard deviation of the four trials below. Trial 1: 49.59 grams Trial 2: 51.25 grams Trial 3: 49.78 grams Trial 4: 50.19 gramsarrow_forwardTo determine the amount of copper and the identity of the copper compound in the unknown sample, a 100-mL solution containing 120.8 mg of the sample was initially prepared. Then, 5.00 mL of this solution was pipetted and diluted with deionized water in another 100-mL volumetric flask. The absorbance of this diluted solution was determined three times at the analytical wavelength. The data is in the table below. Unknown sample Trial 1 Trial 2 Trial 3 Absorbance (AU) 0.388 0.389 0.388 What is the concentration of Cu in analyzed solution (mg/L) for each trial?arrow_forward
- Utilize the structure of Benadryl (Diphenhydramine) shown below to determine its molecular formula. OA.C17H21NO OB. C15H23NO C.C17H23NO OD. C17H11 NO N_arrow_forwardPlease choose an appropriate technique(s) to separate following compounds (it can be one, more than one, on none): Compound Melting Point Density Boiling Point Molar mass Phenol 40.5°C 1.07 g/cm 181.7°C 94.113 g/mol Toluene -95 °C 0.87 g/mL 111 °C 92.141 g/mol Simple Filtration Column Chromatography Distilation Crystallization/Re-Crystallization Separatory funnel None www. MacBook Airarrow_forwardThe TLC and mp data contradict one another with regards to purity. True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY