
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
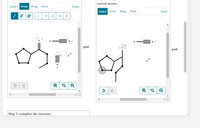
Transcribed Image Text:curved arrows.
Select Draw Rings More
Erase
Select Draw Rings
More
Erase
C
H
N
K
:0 :
N
Q
Step 3: complete the structure.
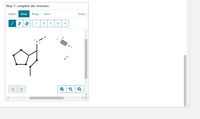
Transcribed Image Text:Step 3: complete the structure.
Erase
Select
Draw
Rings
More
H
N
K
N :
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12) Which structure is different from the compound (I) ? CH₂ CH₂- H H a H CH(CH3)2 H. H CH₂ CH₂ H b -CH(CH₂)2 CH₂ CH₂- H H C H CH(CH₂)2 H7 CH₂ CH₂ CH(CH3)2 darrow_forward1. Predict the products when cyclohexanol is heated in the presence of H+. Show all hydrogen atoms. 2. Predict the products when 1‑propanol is heated in the presence of H+. Include all hydrogen atoms in your structure. Show both the organic product and the inorganic product formed in this reaction.arrow_forwardDraw the skeletal (line-bond) structure of 2- methoxybutan-1-ol. Drawing Version: 1.0.94 + production Atoms, Bonds and Rings Charges Draw or tap a new bond to see smart suggestions. Undo Reset Remove Donearrow_forward
- please help! I know there's a double bond but idk how to get it in there Please help me posting third timearrow_forwardPls help ASAParrow_forwardDraw the structure of the major organic product(s) of the reaction. H3C ● `N H 1. LIAIH4, ether FO 2. H₂O • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forward
- 2. Can you figure out the test reactions for unsaturation (alkene and alkyne)? Please give one example for each test reaction.arrow_forwardDraw the cis and trans isomers of 2-butene, CH, CHCHCH,. Show all hydrogen atoms. cis-2-butene trans-2-butene Select Draw Rings More Erase Select Draw Rings More Erase C H C エarrow_forwardRank the substituents on the asterisked (*) carbon from lowest to highest priority. Select answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b C aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY