
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
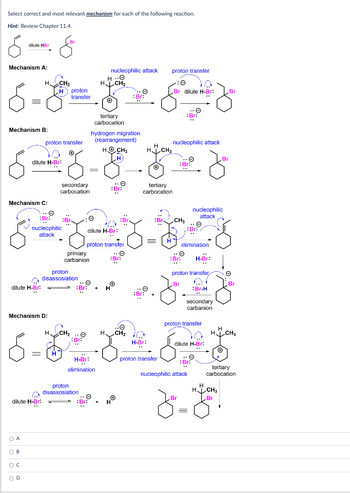
Transcribed Image Text:Select correct and most relevant mechanism for each of the following reaction.
Hint: Review Chapter 11.4.
Mechanism A:
nucleophilic attack proton transfer
H.O
HCH₂
6-8=8²²²6
tertiary
carbocation
hydrogen migration
(rearrangement)
H CH3
H
Mechanism B:
dilute HBr
Mechanism C:
dilute H-Br:
OA
O
O
Mechanism D:
B
dilute H-Br:
C
D
dilute H-Br:
=
CH₂
nucleophilic
attack
Br
H proton
proton transfer
transfer
secondary
carbocation
proton
disassosiation
CH₂
primary
carbanion
Br
=
dilute H-Br:
proton
disassosiation
proton transfer
H-Br:
elimination
H.
Br:
CH₂
H
H CH3
H-Br:
+
tertiary
carbocation
proton transfer
Br dilute H-Br:
nucleophilic attack
:Br
CH₂
elimination
H-Br:
proton transfer,
Br
Br
Br-H
secondary
carbanion
proton transfer
nucleophilic
attack
dilute H-Br:
Bri
nucleophilic attack
=
Br
Br
H
H CH3
H
H₂ CH3
.Br
tertiary
carbocation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. CH3 Ph oto KOH O OH Ethanol Ph Ph-H -CH₂ + KCI H₂O 2. OCOH a = Proton transfer d Radical chain addition g= E2 Elimination b= Lewis acid/base e Electrophilic addition h = SN1 Nucleophilic substitution c = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. HCIarrow_forwardDraw a mechanism for the following reaction: + HO Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron-flow ar an atom, bond, or location where a new bond should be created. H EXP. CONT. OH НО. H+ H C N OSJ - F CI OH Br H+arrow_forward1. aq. NaOH reflux NaCI H20 OH 2. HI a = Proton transfer d = Radical chain addition g = E2 Elimination b = Lewis acid/base e = Electrophilic addition h = SN1 Nucleophilic substitution C = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- 1. H3C H CH3 2. H3C + Br CH3 H3C H excess NH3 NH4CI H H NH2 C2H5 N-C₂H5 C2H5 + C2H5 H-N-C2H5 Br C2H5 a Proton transfer b Lewis acid/base e Electrophilic addition h = Syl Nucleophilic substitution f El Elimination i = SN2 Nucleophilic substitution c Radical chain substitution g E2 Elimination j Electrophilic aromatic substitution d Radical chain addition Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers.arrow_forward10:47 AM Sun Nov 27 ← Question 28 of 41 Draw the Alkyl Halide Rea nt @72% Draw an alkyl halide that produces ONLY the following alkene in an E2 elimination. Ignore any inorganic byproducts. Strong Base Submitarrow_forwardH,SO4 1. H20 n-Bu Na NH2 2. -CN CN 2 Br NaBr NH3 toluene, reflux n-Bu a = Proton transfer d = Radical chain addition g= E2 Elimination b = Lewis acid/base e = Electrophilic addition h = Sy1 Nucleophilic substitution c = Radical chain substitution f=El Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2.arrow_forward
- Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers.arrow_forwardDraw the curved arrows for the mechanism of the following acid-base reaction. NO ₂ + HF → HNO₂ + F Marvin JS :0: ——2=0 Edit drawing H :F: Helparrow_forwardUse the roadmap reactions to determine the steps reactions and the intermediate products for each synthesis. Help with 3&4 only!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY