
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
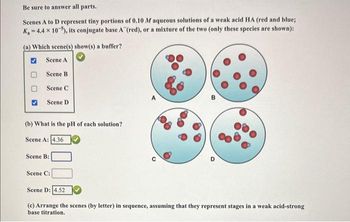
Transcribed Image Text:Be sure to answer all parts.
Scenes A to D represent tiny portions of 0.10 M aqueous solutions of a weak acid HA (red and blue;
K-4.4 x 10-5), its conjugate base A (red), or a mixture of the two (only these species are shown):
(a) Which scene(s) show(s) a buffer?
Scene A
Scene B
Scene
Scene D
(b) What is the pH of each solution?
Scene A: 4.36
Scene B:
Scene C:
Scene D: 4.52
B
(c) Arrange the scenes (by letter) in sequence, assuming that they represent stages in a weak acid-strong
base titration.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I just need assistance with part B 5a) Calculate the pH of a buffer solution that contains 0.79 M NaH2PO4 and 0.13M Na2HPO4 Answer: 6.43 5b) Calculate the change in pH if 0.050 g of solid NaOH is added to 150 mL of the solution in the problem above.arrow_forward5. A buffer solution contains 10.0 mmol of formic acid (HCOOH) and 15.0 mmol of formate (HCO0). If the solution pH is 3.95, then what is the Ka of formic acid? (а) 7.5 х 10-5 (b) 1.1 х 104 (c) 4.1 x 102 (d) 1.7 x 10-4arrow_forward5:48 PM Wed Nov 16 Today 5:48 PM 目。 oint when 15.0 ml. of o. 75% Calculate the pH of a titration at the point when 15.0 mL of 0.15 M NaOH is added to 30.0 mL of 0.20 M HNO3 Edit G ¯¯¯arrow_forward
- What mass (in grams) of sodium acetate (CHCOONa) must be added to 100.00 mL of a 0.115 M solution of acetic acid in order to prepare a buffer solution with a pH of 4.70? The K, of acetic acid is 1.8 x 10-5. Mass of sodium acetate = g ences Mc Graw Hill JUN 27 MacBook Air 000 F1 F2 F3 F4 F5 F6 F7 F8 F9 $ % & 2 4 6. 7 Q W R. Y U < (O 法 # 3arrow_forwardA solution contains 0.312 M HA (K₂ = 2.20-10-7) and 0.680 M NaA. What is the pH of this solution? What is the pH of this solution after 0.212 mol of HCI are added to 1.00 L of this solution? What is the pH of this solution after 0.424 mol of HCI are added to 1.00 L of this solution? Question Help: Video Submit Question [11arrow_forwardThe pH of a 500.00 mL buffer solution containing sodium mandelate and mandelic acid (ka= 3.89x10-4) ((a,=0.90 Please fill in the space with a numerical value with two digits or decimal places without unitsarrow_forward
- This question has multiple parts. Work all the parts to get the most points. a Identify the buffer system(s) - the conjugate acid - base pair(s) - present in a solution that contains equal molar amounts of the following: (Select all that apply.) HC1 KCN | HNO2 KNO2 b (Select all that apply.) OK,CO3 H,CO3 KF HFarrow_forwardA triprotic acid is titrated with KOH. What species are NOT in solution to any large extent between the 1st and 2nd equivalence points? Select all that apply. O H3A O HA? O A³- K+ O OH H2Aarrow_forwardWhat will the pH be when I take a 25 ml aliquot of 0.1023mol/L H3PO4 (The ka values for phosphoric acid are 6.9*10-3, 6.2*10-8 and 4.8*10-13) and add 0.0878 mol/L NaOH in the following amounts? A) 10 ml NaOH B) 30 mL NaOH C) 60 mL NaOH D) 100 mL NaOHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY