
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
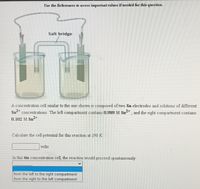
Transcribed Image Text:Use the References to access important values if needed for this question.
Salt bridge
A concentration cell similar to the one shown is composed of two Sn electrodes and solutions of different
Sn-* concentrations. The left compartment contains 0.989 M Sn2+, and the right compartment contains
0.102 M Sn2+
Calculate the cell potential for this reaction at 298 K.
volts
In this tin concentration cell, the reaction would proceed spontaneously
from the left to the right compartment
from the right to the left compartment
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Write the cell notation based on the description of the following information: Iron (III) ions are reduced to iron (II) ions in the presence of platinum electrode, and tin is oxidized to tin (II) under standard conditions.arrow_forwardA certain metal M forms a soluble nitrate salt M (NO3)2. Suppose the left half cell of a galvanic cell apparatus is filled with a 3.00 M solution of M (NO3), and the right half cell with a 30.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 20.0 °C. Which electrode will be positive? O left O right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. 0arrow_forwardA certain metal M forms a soluble nitrate salt M(NO3)2. Suppose the left half cell of a galvanic cell apparatus is filled with a 1.50 M solution of M(NO3)₂ and the right half cell with a 15.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 30.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. left right X Ś ?arrow_forward
- A certain metal M forms a soluble nitrate salt MNO,. Suppose the left half cell of a galvanic cell apparatus is filled with a 3.50 M solution of MNO, and the right half cell with a 17.5 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 30.0 °C. left x10 Which electrode will be positive? right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardAnswer the following questions using the data in Appendix L of the textbook. a. Calculate the standard cell potential for a galvanic cell made with Ni and Ag. b. Calculate the standard cell potential for a galvanic cell made with Ni and Zn.arrow_forwardIn this aluminum concentration cell, the reaction would proceed spontaneously (from the left to the right compartment or right to left compartment)arrow_forward
- In an electrochemical cell, a metal anode lost 0.286 g while a total volume of 0.04854 L of hydrogen gas was produced. The temperature was 298 K and the barometric pressure was 763.5 mm Hg. At 298 K, the vapor pressure of water is 23.8 mm Hg. What is the molar mass of the metal?arrow_forwardDetermine the ΔG° in KJ for a voltaic cell made of a Ag strip and a Pb strip immersed in 1.0 M aqueous AgNO3 and Pb(NO3)2 solutions, respectively, with all other cell components in place. (Use Table 18.3 for your standard cell potentials.) Determine the thermodynamic equilibrium constant K for a voltaic cell made of a Zn strip and a Pb strip immersed in 1.0 M aqueous Zn(NO3)2 and Pb(NO3)2 solutions, respectively, at 25.00°C, with all other cell components in place. (Use Table 18.3 for your standard cell potentials.)arrow_forwardSalt bridge A concentration cell similar to the one shown is composed of two Co electrodes and solutions of different Co²+ concentrations. The left compartment contains 0.819 M Co²+, and the right compartment contains 1.27 M Co²+ Calculate the cell potential for this reaction at 298 K. volts In this cobalt concentration cell, the compartment on the left is the and the compartment on the right is thearrow_forward
- bew DARGERS Determine the thermodynamic equilibrium constant K for a voltaic cell made of a Ag strip and an Al strip immersed in 1.0 M aqueous AgNO3 and Al(NO3)3 solutions, respectively, at 25.00°C, with all other cell components in place. (Use Table 18.3 for your standard cell potentials.) Type your numeric answer and submit Hint Equation 18.8 in Section 18.3.1 relates cell voltage to AG. SOPINIOANE Cat 0 Cannot be empty HE HIG Xarrow_forwardConsider the following galvanic cells. Diagram I Au- cº 1.0 M Au³+ 3+ chemPad XX² (b) balanced cell equation II chemPad 8⁰ XX→→ 1.0 M Cu+ 1.0 M Cu²+ For each galvanic cell, give the balanced cell reaction and determine °. Standard reduction potentials are found in the Standard Reduction Potentials table. (Use the lowest possible whole number coefficients. Include states-of-matter under the given conditions in your answer.) (a) balanced cell equation I Help Greek Pt Help Greek Diagram II Cd- 1.0 M Cd2+ -Pt Ai 1.0 M VO₂, 1.0 MH+ 1.0 M VO²+arrow_forwardA certain metal M forms a soluble nitrate salt M(NO3)2. Suppose the left half cell of a galvanic cell apparatus is filled with a 2.50 mM solution of M (NO3)2 and the right half cell with a 5.00 M solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 20.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. Π left x10 rightarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY