
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
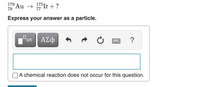
Transcribed Image Text:179
79
Au → 7Ir + ?
Express your answer as a particle.
0.
ΑΣφ
?
A chemical reaction does not occur for this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 15 Match the correct prefix with the factor. in A. 10-9 k B. 103 C. 102 D. 109 E. 10-2 F. 103arrow_forwardClassify each substance as a pure substance, a homogeneous mixture, or a heterogeneous mixture.a. air c. soil e. sedimentb. aerosol d. water f. muddy waterarrow_forward2. Homogeneous mixtures(s) A. distilled water B. gasoline C. sandy water D. copper turnings + iron filings E. white gold (gold + palladium)arrow_forward
- 0:56 C X Get ready for the Computer Marked A... Which of the structures below better matches the provided 13C-NMR spectrum? T || 200 180 160 140 120 100 PPM 80 60 40 20 H H H... C. HO, CI H. H. H. O. CH3 В CI H. Save and Close Submit 191.0 140.1 135.0arrow_forwardWhich is considered an elemental substance? A.CO (carbon monoxide) B.H2O (water) C.I2 (iodine) D.A and C are elemental substances. E.All of the above are elemental substances.arrow_forwardSignifi Bb My Bla Digital ng.cen Week Submit Answer HOME References Chemi ploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707785886&snaps... Min Bb NCBM g Use the References to access important values if needed for this question. G The st ✰ C Q Search this The liquid 1-chloropropane has a density of 0.890 g/mL at 20 °C. If a sample of this liquid at 20 °C has a volume of 1.97 L, how many grams of liquid are there in the sample?arrow_forward
- Which of the following is a heterogenous mixture? A. raisin bran B. milk C. coffee D. air E. sugar waterarrow_forwardwhat is an atom? What is it characteristics? How does an atom live and exists in the biosphere?arrow_forwardWhat is the classification of the substance in the figure? A. element B. homogeneous mixture C. compound D. heterogeneous mixturearrow_forward
- Apps M (28) - r.a... ɔlgl y University in Dubai... تسجيل الدخول - جام. . . D YouTube Maps M Gr Mc Graw Hill O of 27 Concepts completed Multiple Select Question Select all that apply Select all of the following that are characteristics of matter. O Takes up space Has mass O Always visible to the human eye O Present only in a solid form Need help? Revlew these concept resources.arrow_forward23/ 25 The following set of data is described as (assume X₂-190): 168, 200, 153, 169 and 162 ppm a. Accurate and precise. b. Accute but not precise. c. Not accurate but precise. d. Not accurate and not precise. e. It is precise but difficult to judge the accuracy.arrow_forwardg shape, color, Choose all that apply. A. The property can be observed without changing the identity of the substanca B. The property depends on the amount of the substance present. C. The state of the substance changes when you measure the property. D. The property can be observed or measured. EVIDENCE NOTEBOOK 19. How do the physical properties of the rocks at the beginning of the lesson differ? Are there physical properties that might differ, but that you cannot observe in the photos? Record your evidence. Lesson 1 The Propertiesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY