
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
For the protons what are the splitting patterns of each?
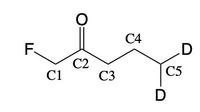
Transcribed Image Text:C4
F
.D
C2
C1
C3
C5
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (1)Draw a ground state energy level diagram for a silicon atom that shows where all of the electrons would be (n, L, ml and show spin up or spin down with an arrow). (2) Determine the values of L and S for the ground state using Hund's rule. (3) For chlorine, repeat steps 1 and 2.arrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardFor each of the statements below, indicate whether it is true or false and explain your reasoning. (1-3 sentences) ( 1/ It takes more energy to ionize an electron from the 2s orbital than an electron from the 2p orbital in the Li2+ ion. 2/ The electron affinity of the Ne atom is larger than the electron affinity of the F atom. 3/ K* has a larger radius than Ar. 4/ For a diatomic molecule, in which the internuclear axis is the z-axis, the 3dz2 orbital cannot mix with the 2px orbital. 5/ The internuclear distance of O2 increases as it takes an additional electron to become O2.arrow_forward
- stion 3 yChart - Sch... Home - Profession... Enroll Enr 15 pts To preview image click here For the following anion choose the correct options for the following questions. 1. Choose the correct resonance contributors ✓ [Select] A and B B and F C and E ct representation of wave A and F [Select] 3. Choose the correct representation of wave function of LUMO [Select]arrow_forwardQuestion 3 What is wrong with the following orbital spin diagram? tt 1s One arrow should be in the 1s and one arrow should be in the 1p. One arrow should be pointing up and one arrow should be pointing down. O The 1s has two orbitals so one arrow should be in the first 1s orbital and the other arrow should be in the second 1s orbital. O Both arrows should be pointing down, not up.arrow_forwardHello! I need help, please. See picturearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY