
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
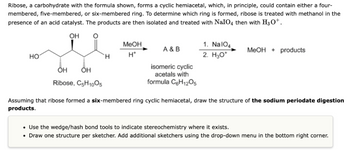
Transcribed Image Text:Ribose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-
membered, five-membered, or six-membered ring. To determine which ring is formed, ribose is treated with methanol in the
presence of an acid catalyst. The products are then isolated and treated with NaIO4 then with H3O+.
OH
HO
OH
OH
Ribose, C5H10O5
H
MeOH
H*
A & B
isomeric cyclic
acetals with
formula C6H₁2O5
1. NalO4
2. H₂O*
MeOH products
Assuming that ribose formed a six-membered ring cyclic hemiacetal, draw the structure of the sodium periodate digestion
products.
• Use the wedge/hash bond tools to indicate stereochemistry where it exists.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH₂CH3 + HBrarrow_forwardThe carbohydrate shown is a (an): но OH OH Triose tetrose pentose hexose heptosearrow_forwardHow much lactic acid (C3H6O3) is produced when 225g glucose (C6H12O6) is used as a substrate in fermentation. Assuming that the sugar is completely fermented to lactic acid.arrow_forward
- Draw the cyclic forms of beta-1,3'-d-ditalosearrow_forward1) The starch in 25kg (~1 bushel) of corn is hydrolyzed and in the process 1.8kg of water (H2O) is consumed. How many moles of glucose (C6H12O6) would you expect to be produced? C6H12O6 -> 2 CO2 + 2 C2H5OH. 2) If the glucose from the above hydrolysis is fermented, how many moles of ethanol (C2H5OH) would result? How many grams? 3) If ethanol has a density of 0.789g/ml, what would the volume of the ethanol be in ml? What would the volume be in gallons (1gal = 3785ml)? 4) The above ethanol could be converted to Vodka by diluting to 50% ethanol (adding enough water to double the volume). What would the volume of the resulting Vodka be in ml? If the volume of a “shot” of Vodka is 30ml, how many shots of vodka could be produced from a bushel of corn?arrow_forwardRibose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. When D-ribose is treated with methanol in the presence of an acid catalyst, two cyclic acetals, A and B, are formed, both with molecular formula C,H,0, These are separated, and each is treated with sodium periodate (Section 10.8C) followed by dilute aqueous acid. Both A and B yield the same three products in the same ratios. он о CHO СНО H+ CH,OH A +B ÕH 1. NalO, 2. H,0* НО CHO + CHOH + CH,OH ÕH CH,OH Isomeric cyclic acetals with molecular formula CH12O, D-Ribose (C;H1605) From this information, deduce whether the cyclic hemiacetal formed by D-ribose is four- membered, five-membered, or six-membered.arrow_forward
- Draw the structure of the repeating unit of the polyamide formed from this reaction. CI H H₂N NH₂ You do not have to consider stereochemistry. + CI polymerizationarrow_forwardIdentify the organic functional group and reaction type for the following reaction. The reactant is a(n) - carboxylic acid hexose - Aldohexose - aldotetrose -deoxyhexose -carboxylic acid tetrose - ketohexose The product is a(n) - carboxylic acid tetrose - aldotetrose -alcohol hexose -aldohexose -carboxylic acid hexose - alcohol tetrose The reaction type is - hemiacetal formation -hydrolysis -oxidation( Benedict’s) -acetal formation -reduction( hydrogenation) - mutarotationarrow_forwardSugar alcohols (alditols) are formed by chemically reducing aldoses or ketoses. Which one of the following sugars will give a negative Benedict's Test? aldopentoses alditols 2- Ketopentoses an aldose undergoing mutarotation in solutionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY