
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%

Transcribed Image Text:Rewrite this measurement with a simpler unit, if possible.
2
g mL g
1.3
2
g mL•mL•s
Note: If you can simplify the unit at allI, it may be possible to make more than one simplification. Be sure your final answer uses
the simplest possible unit.
x10
?
미
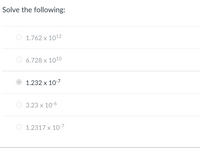
Transcribed Image Text:Solve the following:
1.762 x 1012
6.728 x 1010
1.232 x 10-7
3.23 x 10-6
O 1.2317 x 10-7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H2O). Suppose 73.6 g of hydrobromic acid is mixed with 19. g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits. g ☐ x10 ☑arrow_forwardA student prepares a 0.38 mM aqueous solution of acetic acid (CH3CO₂H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. 11 % 0.0 x10 X S 0 Earrow_forwardA certain liquid X has a normal boiling point of 105.70°C and a boiling point elevation constant =Kb2.16·°C·kgmol−1. A solution is prepared by dissolving some potassium bromide (KBr) in 400.g of X. This solution boils at 107.3°C. Calculate the mass of KBr that was dissolved. Round your answer to 2 significant digits. ____garrow_forward
- Convert the following measurement. -5 kg, g 7.6 x 10 x10 mol-dL mol·L Xarrow_forwardA chemist adds 305.0 mL of a 0.41 M barium chloride (BaCl₂) solution to a reaction flask. Calculate the millimoles of barium chloride the chemist has added to the flask. Round your answer to 2 significant digits. 1.25 x 10 mmol [NN] X 5 ? Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Ad DII F11 F12 F10 FO FB F7 esc ! 1 Explanation 2 Check 39: F2 14/ # 3 80 F3 $ 4 000 F4 R % 5 ܐ FS T ^ 6 F6 Y & 7 U * 00 8 ہ ۔ 9 ) 0 0 P + 11 T D olo Ararrow_forwardEarth's oceans have an average depth of 3800 m, a total area of 3.63 x 10 km², and an average concentration of dissolved gold of 5.8 × 10. If a recent $987.00 troy oz what is the value of gold in the oceans? (I troy oz 31.1 g) Round your answer to 2 significant figures. Note: Reference the SI prefixes and Unit conversions for derived SI units tables for additional information. price of gold was 0arrow_forward
- A chemist adds 1.05 L of a 2.20 mol/L silver nitrate (AgNOs) solution to a reaction flask. Calculate the moles of silver nitrate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.arrow_forwardAqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O . Suppose 28. g of hydrochloric acid is mixed with 53.0 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardGaseous ethane (CH3 CH3) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 13.8 g of ethane is mixed with 20. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g x10 X Sarrow_forward
- Aqueous sulfuric acid H2SO4 will react with solid sodium hydroxide NaOH to produce aqueous sodium sulfate Na2SO4 and liquid water H2O . Suppose 4.90 g of sulfuric acid is mixed with 5.1 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.arrow_forwardThe chemical formula for hydrogen bromide is HBr. A chemist measured the amount of hydrogen bromide produced during an experiment. She finds that 5.9 g of hydrogen bromide is produced. Calculate the number of moles of hydrogen bromide produced. Be sure your answer has the correct number of significant digits. 477. mol x10 X S 4arrow_forward1) State your interpretation of the meaning of the slope for the first graph you prepared of Volume of Steel Nuts (mL) versus Number of Steel Nuts. Hint: Understanding the meaning of a slope involves several factors: the units which are a ratio of the y axis units over the x axis units, the interpretation of the slope as “number of the y axis units for every one of the x axis units” and the concept that constructing the best line or curve that follows the trend in the data is a sort of averaging of the random error throughout the data.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY