
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
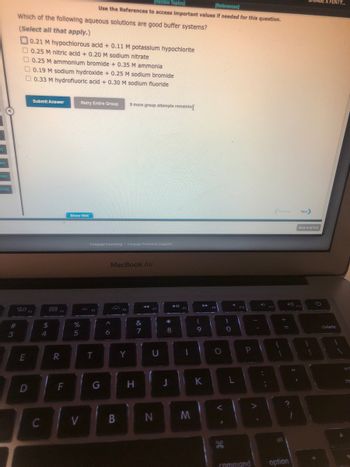
Transcribed Image Text:**Buffer System Identification in Aqueous Solutions**
**Question:**
Which of the following aqueous solutions are good buffer systems? *(Select all that apply.)*
1. \(0.21 \, \text{M hypochlorous acid} + 0.11 \, \text{M potassium hypochlorite}\)
2. \(0.25 \, \text{M nitric acid} + 0.20 \, \text{M sodium nitrate}\)
3. \(0.25 \, \text{M ammonium bromide} + 0.35 \, \text{M ammonia}\)
4. \(0.19 \, \text{M sodium hydroxide} + 0.25 \, \text{M sodium bromide}\)
5. \(0.33 \, \text{M hydrofluoric acid} + 0.30 \, \text{M sodium fluoride}\)
**Submission Options:**
- **Submit Answer**: Finalize and submit your selected responses.
- **Retry Entire Group**: Reset all choices and attempt again.
**Note:**
You have 9 more group attempts remaining.
**Instructional Hint:**
Use the provided references to access key values if needed to determine buffer systems.
**Explanation:**
Buffers consist of a weak acid and its conjugate base or a weak base and its conjugate acid. Identify which combinations meet this criterion.
**Meta:**
This information is used during the learning process to help students understand and identify chemical buffer systems effectively.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- which of the following titrations result in a basic solution at equivalence point ?arrow_forwardName the two parts of an atom, where you can find the following subatomic particles (protons, neutrons, and electrons).arrow_forwardWhich of the following aqueous solutions are good buffer systems?(more than one answer maybe required) a) 0.40 M sodium bromide + 0.30 M calcium bromide b) 0.25 M ammonia + 0.31 M barium hydroxide c) 0.14 M hypochlorous acid + 0.17 M sodium hypochlorite d) 0.25 M hydrochloric acid + 0.23 M potassium chloride e) 0.14 M potassium hydroxide + 0.24 M potassium chloridearrow_forward
- Use the following information as a guide to help answer the next three questions. Your answer MUST be in the same format. Indicators can be used to help approximate the pH of a solution based on the indicator colour. Here is an example of how to word the pH approximation for bromothymol blue. Indicator Colour pH approximation Yellow 6.0 and below Green between .0 and 7.6 6. Blue 7.6 and above A Chemistry experiment is done where the pH of various solutions are tested, using indicators. The colours of indicators are recorded as shown below. Using the method outlined above in the example of bromothymol blue, indicate what the colours of the indicators tell us about the pH approximation of the solution. Values and wording are very important, so be precise. Be sure to word your pH approximation as outlined in the example. Indicator Colour pH approximation methyl red orange Indicator Colour pH approximation phenolphthalein colourless Indicator Colour pH approximation phenol red redarrow_forwardSelect ALL of the following that can be determined from the titration of a strong, monoprotic acid with a standardized solution of sodium hydroxide? moles of acid in the titration moles of hydroxide ions used in the titration mass of acid titrated | volume of sodium hydroxide used in the titration Select ALL of the following quantities that must be used in a titration calculation to find the identity of an unknown strong acid: concentration of the standardized base solution stoichiometric ratio of acid to base volume of the acid solution used in the titration volume of the base used in the titration at the equivalence point Need Help?arrow_forwardWhat chemical would have to be in the same solution as CHO2- in order to form a buffer? Question 9 options: CO2- O2- HCHO2 CO22-arrow_forward
- Question Completion Status: For Solution A, approximately how many mL of HCI were needed to exceed the buffering capacity of this solution? Enter the number only. Don't enter units as part of your answer and don't use commas or scientific notation. Titration curve of Solution A 14 13 12 11- 10 9 8 pH 7 6 5 4 3 2 1 5 4 3 2 1 HCI added (mL) 0 1 2 3 4 5 NaOH added (mL) 14 13 12- 11 10 9 8 pH 7 6.8 2 65 4 3 2 1 Titration curve of Solution B Click Save and Submit to save and submit. Click Save All Answers to save all answers. 54 32 1 HCI added (mL) 0123 NaOH added (mL)arrow_forwardWhich solution would make an effective buffer? Solution 1: 1.0 M HCI and 1.25 M NaOH Solution 2: 1.0 M NAHCO3 and 1.0M NANO3 Solution 3: 1.0 M HC2H3O2 and 1.0 M NAC2H3O2 Solution 4: 1.0 M NH3 and 1.0 M HC2H3O2arrow_forwardIn an acid-base titration experiment, the equivalence point on the titration curve was at a pH of 6.0. Based on this information, which of the following is represents the titration experiment that was conducted? It was a titration conducted between a strong base and strong acid It was a titration conducted between a weak acid and a strong base. It was a titration conducted between a strong acid and a weak basearrow_forward
- define the buffer capacity. a) buffer capacity is the amount of acid that can be added until all of the acid is used up b) buffer capacity is the amount of base that can be added until all of the base is used up c) buffer capacity is the amount of acid that can be added until all of the base is used up d) buffer capacity is the amount of acid ir base that can be added to a buffer without destroying its effectiveness e) buffer capacity is the amount of base that can be added until all of the acid is used uparrow_forwardMixing which of the following pairs will make a buffer solution? O CsF, HF O KBr, HBr O Nal, HI RbCl, HC1arrow_forwardAn aqueous solution of an unknown solid salt was placed into five different test tubes. Into each test tube a couple of drops of different indicators were then added to each test tube and the color of the indicator in the solution was recorded as shown below. What is the approximate pH of the solution? Test pH Acid Base Color in Unknown Indicator tube Range Color Color Solution 3.0 - 1 Methyl orange 4.4 Red Yellow Yellow 3.0 - Congo Red Purple Red Red 5.0 Bromcresol 3.8 - Yellow Blue Blue Green 5.4 Bromthymol 6.0 - 7.6 4 Yellow Blue Yellow Blue 8.2 - Phenolphthalein Colorless Blue Colorless 10.0 4.3 O 8.4 O 5.2 6.7arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY