
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![Resonance: Resonance form of ethyl propanoate
Part A
Draw the resonance structure of the following substance.
Interactive 3D display mode
H₂C
NN
[1]
CH₂
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single
bond is active by default.
A
7
L
H2D EXP CONT.
L
1
Marvin JS
by ChemAxon
H
C
N
O
S
CI
Br
-
5 of 26
P
Review | Constants | Periodic Table](https://content.bartleby.com/qna-images/question/62cc748c-628b-4ab0-8610-e208833b302f/1c7aa6f4-0d2b-4dc4-a06e-59da978a9283/slljvzu_thumbnail.jpeg)
Transcribed Image Text:Resonance: Resonance form of ethyl propanoate
Part A
Draw the resonance structure of the following substance.
Interactive 3D display mode
H₂C
NN
[1]
CH₂
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single
bond is active by default.
A
7
L
H2D EXP CONT.
L
1
Marvin JS
by ChemAxon
H
C
N
O
S
CI
Br
-
5 of 26
P
Review | Constants | Periodic Table
Expert Solution
arrow_forward
Step 1
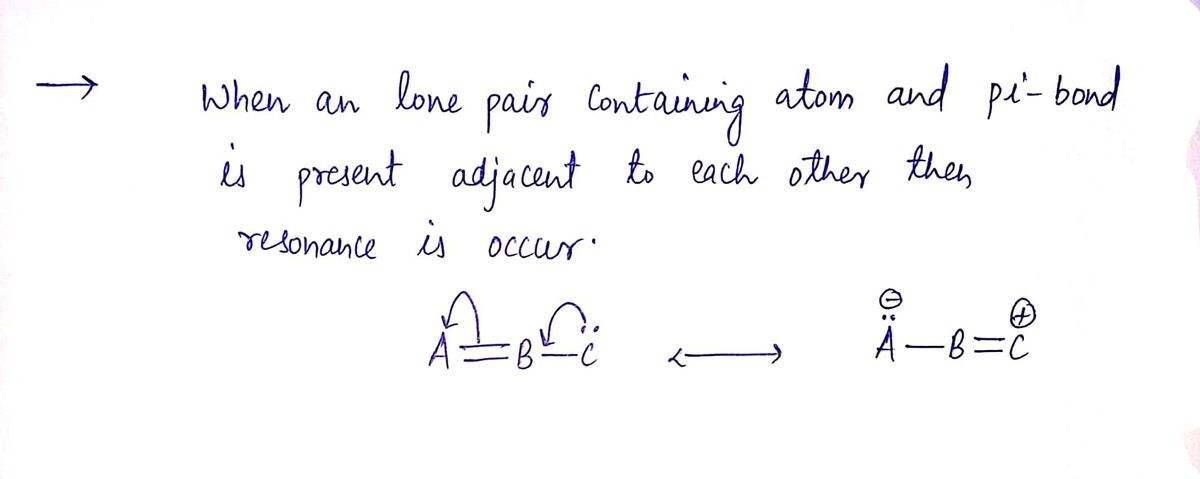
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8) Provide the best resonance structures of the molecules shown below. Place a circle(s) around the best resonance structure(s) for each molecule also considering the initial structure given. a) b) OH HN OH OH ↓ ↑arrow_forwardBased on the arrows already drawn, draw the resonance structures (making sure to include any charges), and highlight which structure is most important/present for each molecule صدار H N [2 I ]arrow_forwardDraw the structure of CH4, COH4, COH2, AND CO2H2arrow_forward
- Show three or four different drawings that show three or four different relevant resonance structures for the compound shown below. Be careful to use correct arrows when that show electron movements between each resonance shown Also, draw the hybrid. NH Oarrow_forwardNonearrow_forwardDraw a second resonance form for the structure shown below. ö: H3C-N :ö:arrow_forward
- Draw at least 10 more resonance structures for acetaminophen, the active pain reliever in Tylenol.arrow_forwardDirections: Read the following statements. Write your answer in a separate sheet of paper. Lewis dot ion after electron symbol of each ion if ionic bond is formed Charge of each Lewis dot Туре of Bond Atoms symbol of each atom Formula of the transfer if ionic involved Product bond is formed Na, O Са, N S, CIarrow_forward2. Draw possible resonance structures where necessary? If no resonance structures are possible indicate why. Show formal charge in the resulting structures. Show all steps. N :0 ✓ Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY