
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:!
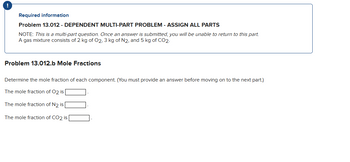
Required information
Problem 13.012 - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
A gas mixture consists of 2 kg of O2, 3 kg of N2, and 5 kg of CO2.
Problem 13.012.b Mole Fractions
Determine the mole fraction of each component. (You must provide an answer before moving on to the next part.)
The mole fraction of O2 is
The mole fraction of N2 is
The mole fraction of CO2 is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ⠀ M Apple Google Disney ESPN Yahoo! ☆ Kc = prod03-cnow-owl.cengagenow.com Biomedical Careers Program Apple iCloud ☆ B = Yahoo G G D2L Images Bing Google Wikipedia Facebook C COWLv2 |... D2L A student ran the following reaction in the laboratory at 702 K: N₂(g) + 3H₂(g) → 2NH3 (9) Twitter LinkedIn The Weather Channel Yelp b b c TripAdvisor C G M G с When she introduced 0.0328 moles of N₂ (g) and 0.0676 moles of H₂(g) into a 1.00 liter container, she found the equilibrium concentration of NH3(g) to be 0.00153 M. Calculate the equilibrium constant, Kc, she obtained for this reaction. + 88 Use the References to access Marrow_forwardHello I need help with the following problemarrow_forwardNeed BEER'S LAW WITH FULL STEPS BY STEPS EXPLANATION DERIVE LAW. OK. NOTE: NO BEERS LAMBERT LAW. ONLY BEERS LAW DEFINITION INTRODUCTION DERIVATION FULL OK. ONLY HUMAN EXPERT SOLVE IT NOT AI . Only Handwritingarrow_forward
- roduced s under ana-icu-M, his expressions that are not correct, provide the correct expression along with an explanation. a. 2NBr3(s) = N₂(g) + 3Br22(g), K = [N₂][Br₂]3³ [NBr3]2 = b. CuO(s) + H₂(g) = Cu(1) + H₂O(g), K = c. 4Fe(s) + 302(g) = 2Fe₂O3(s), K = 1 [0₂]³ [Cu] [H₂O] [H₂] 1arrow_forwardI need help with 15.37arrow_forwardAs you are walking across your laboratory, you notice a 5.25 L flask containing a gaseous mixture of 0.0205 mole NO2 (9) and 0.750 mol N2O4 (q) at 25°C. 4 (g) Is this mixture at equilibrium? If not, will the reaction proceed towards forming more products, or more reactants? N2O4 4 (9) → 2NO2 (9) Ko = 4.61 x 103 at 25°Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY