
Please help answer the question regarding heat transfer. I tried solving it and came up with 152.56 oC but it says that it is incorrect. Please show how to get the correct answer. Thank you.
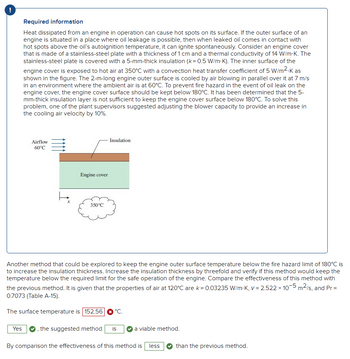
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

It says surface Temperature is not 125.49oC (look at picture I attached). What would be the correct value? Thank you.
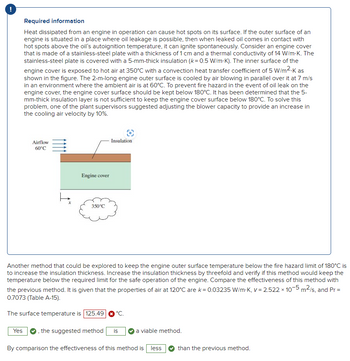
It STILL says the answer is wron (see attached photo). Please provide correct answer. Thank you.
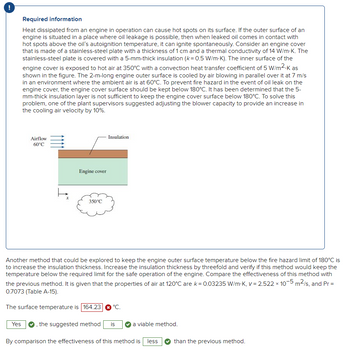
It still says the answer is wrong. Can you please solve for the correct answer? Thank you.
It says surface Temperature is not 125.49oC (look at picture I attached). What would be the correct value? Thank you.

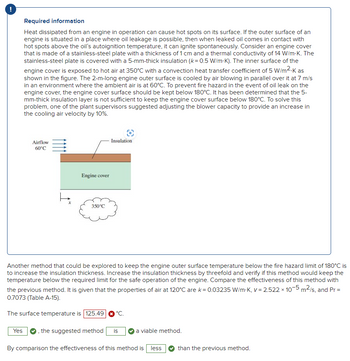
It STILL says the answer is wron (see attached photo). Please provide correct answer. Thank you.

It still says the answer is wrong. Can you please solve for the correct answer? Thank you.

- I just seen a specific heat question where the notation was given in kJ/kg¹ K¹ (exponent is negative), just looking for explanation.arrow_forwardplease solve and explain and show all step in solutionarrow_forwardThis is a repeated question. I have asked this question but it was rejected. I am submitting the problems to check my understanding. I completed the problems already and I am not being graded on them. If you are able to, please state your assumptions and table.arrow_forward
- How much mass of aluminium materials required (in kg) with a specific heat capacity of 0.82 kJ/KgK that absorbs the heat energy of 592 kJ and it has a temperature change of 62 K.arrow_forward1. Nitrogen at 200 psig is used to fill tank of 120 in³. The filling process is very slow and the contents of the tank attain 73°F. Find the mass of nitrogen, in Ib, inside the tank.arrow_forwardAnswer the following questions, in the provided space in the table: Question Solution & Answer 1. A l-m' rigid tank contains 10 kg of water (in any phase or phases) At 160'C. The pressure in the tank is ? 2. What is the boiling temperature Of water at 175 kPa? 3. What is the state of H.O at 1554.9 kPa and 200 C? 4. What is the state of water at 300 C and 0.2 MPa? 5. What is the state of water at 0.01 C and 0.5 kPa? 6. What is the triple point? 7. What is the density of Compressed liquid-water at 500 kPa and 100 C? 8. What is the amount of heat required to evaporate 1 kg of saturated liquid water at 1000 kPa?arrow_forward
- Do not make any kind of mistakes in the solution.arrow_forwardLiquid nitrogen of 10 lbm mass is located inside the container under the 350psi pressure at the saturated liquid stage. It was a hot day and the technician forgot to take the container to the lab. The pressure was gradually increased due to heating the container. At some time, the technician noticed the pressure gage reading is increased to 450 psi and he rushed to hide the container to the lab to cool it down. Draw the process using the phase envelop in T-V scale. Show all stages and denote by arrow the process. Using NIST webbook determine: The initial density of the liquid nitrogen in kg/m3. The volume in m3 of the liquid nitrogen inside the container. The initial temperature in C inside the container. What is the temperature in C at 450psi? Is nitrogen still at the liquid stage? Why do you think so?arrow_forwardOn some temperature scale 0oC is equivalent to 80oV and 100oC is equivalent to 250oV. Determine the temperature in oC corresponding to 200oV.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





