
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Determine the structure based on the following spectrum:
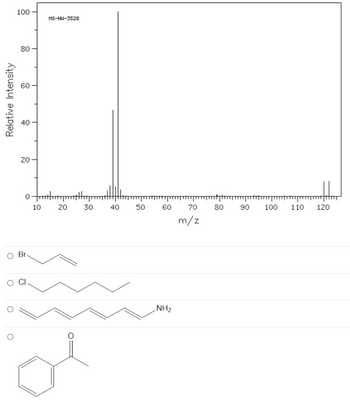
Transcribed Image Text:### Mass Spectrometry Analysis: Identification of Organic Compounds
#### Introduction
Mass spectrometry (MS) is a powerful analytical technique used to identify unknown compounds, determine the structure and chemical properties of molecules, and quantify the amount of a substance. The graph presented here is a mass spectrum, which plots the relative intensity of detected ions against their mass-to-charge ratio (m/z).
#### Description and Analysis of the Mass Spectrum (ID: MS-NW-3528)
The mass spectrum provided displays peaks indicating the presence of ions with specific mass-to-charge (m/z) ratios.
- **X-Axis (m/z):** This axis represents the mass-to-charge ratio of the ions detected.
- **Y-Axis (Relative Intensity):** This axis indicates the relative abundance of each detected ion as a percentage of the most intense ion (base peak).
Key observations:
- A very prominent peak at **m/z 41**, which is the base peak, representing the most stable and abundant ion fragment.
- Several smaller peaks between **m/z 30 and 50**.
- Smaller peaks observed at **m/z 79 and 81**, suggesting the presence of isotopic variants.
- Peaks at higher m/z values around **m/z 120** and higher, though much less intense.
#### Possible Compound Identification
Given this mass spectrum, you are provided with four chemical structure options to identify the unknown compound:
1. **Bromoalkene:**
- Contains a bromine atom.
- Look for peaks corresponding to characteristic bromine isotope patterns (approximately m/z 79 and 81).
2. **Chloroalkane:**
- Includes a chlorine atom.
- Peaks would appear for chlorine isotopes at m/z approx. 35 and 37.
3. **Aminopolyene:**
- Contains an amine group (-NH2) and a polyene structure.
- Multiple peaks due to fragmentation patterns of a polyene chain.
4. **Aromatic Ketone:**
- Comprises a benzene ring and a ketone functional group.
- Look for a peak at m/z 105 (phenyl cation) and other characteristic aromatic fragments.
#### Summary
To identify the compound based on the provided mass spectrum, analyze the peaks and match them with the known fragmentation patterns of the provided chemical structures. Consider isotopic patterns and the molecular weight of each compound to infer the correct match.
**Note:**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- FOR EACH SPECTRUM BELOW, • IDENTIFY (ASSIGN) THE MOLECULAR ION PEAK AND THE MOLECULAR WT OF THE COMPOUND. ( KEEP IN MIND THAT SOMETIMES THE MOLECULAR ION MAY NOT BE PRESENT, ESPECIALLY FOR LARGE AND BRANCHED CHAIN MOLECULES WITH NO RINGS SINCE THEY CAN FORM STABLE CATION FRAGMENTS) • IDENTIFY THE BASE PEAK AND DRAW STRUCTURE FOR IT ( ASSIGNING THE PEAKS).) • ASSIGN STRUCTURES FOR OTHER PROMINENT FRAGMENTS IF POSSIBLE • BASED ON THIS, SUGGEST A STRUCTURE FOR THE COMPOUND THAT IS CONSISTENT WITH THE DEGREE OF UNSATURATION OF THE COMPOUND INDICATE THE BONDS THAT FRAGMENT, RESULTING IN THE OBSERVED MASS SPECTRAL FRAGMENTSarrow_forwardCounterfeit drugs are a common problem in developing regions of the world. Oftentimes, counterfeit pills are made with compounds such as lactose. A lab technician has obtained the IR spectrum shown above for a sample reported to be citalopram, an antidepressant drug. Does the IR spectrum belong to citalopram or lactose? Explain your answer by describing what feature of the IR spectrum confirms your choice and describe what feature is missing from the IR spectrum for the other compound. A. citalopram B. lactosearrow_forwardHow does the operating frequency in NMR spectroscopy compare with the operating frequency in IR and UV/Vis spectroscopy?arrow_forward
- FOR EACH SPECTRUM BELOW, • IDENTIFY (ASSIGN) THE MOLECULAR ION PEAK AND THE MOLECULAR WT OF THE COMPOUND. ( KEEP IN MIND THAT SOMETIMES THE MOLECULAR ION MAY NOT BE PRESENT, ESPECIALLY FOR LARGE AND BRANCHED CHAIN MOLECULES WITH NO RINGS SINCE THEY CAN FORM STABLE CATION FRAGMENTS) • IDENTIFY THE BASE PEAK AND DRAW STRUCTURE FOR IT ( ASSIGNING THE PEAKS).) • ASSIGN STRUCTURES FOR OTHER PROMINENT FRAGMENTS IF POSSIBLE • BASED ON THIS, SUGGEST A STRUCTURE FOR THE COMPOUND THAT IS CONSISTENT WITH THE DEGREE OF UNSATURATION OF THE COMPOUND INDICATE THE BONDS THAT FRAGMENT, RESULTING IN THE OBSERVED MASS SPECTRAL FRAGMENTSarrow_forwardWhich of the following compound is consistent with the following 13CNMR spectrum? To preview the image click here 80 до A ОН 70 60 ОН B 50 40 PPM 30 Хон 20 10 ОН D оarrow_forwardFOR EACH SPECTRUM BELOW, • IDENTIFY (ASSIGN) THE MOLECULAR ION PEAK AND THE MOLECULAR WT OF THE COMPOUND. ( KEEP IN MIND THAT SOMETIMES THE MOLECULAR ION MAY NOT BE PRESENT, ESPECIALLY FOR LARGE AND BRANCHED CHAIN MOLECULES WITH NO RINGS SINCE THEY CAN FORM STABLE CATION FRAGMENTS) • IDENTIFY THE BASE PEAK AND DRAW STRUCTURE FOR IT ( ASSIGNING THE PEAKS).) • ASSIGN STRUCTURES FOR OTHER PROMINENT FRAGMENTS IF POSSIBLE • BASED ON THIS, SUGGEST A STRUCTURE FOR THE COMPOUND THAT IS CONSISTENT WITH THE DEGREE OF UNSATURATION OF THE COMPOUND INDICATE THE BONDS THAT FRAGMENT, RESULTING IN THE OBSERVED MASS SPECTRAL FRAGMENTSarrow_forward
- A total of how many signals are expected in the ¹H spectrum of the given compound? CI оarrow_forwardDraw the structure of the compound whose spectroscopy data is provided. CoH120 2.6 ppm, 1H, sextet 2.1 ppm, 3H, singlet 1.7 - 1.3 ppm, 2H, multiplet 1.1 ppm, 3H, doublet 0.9 ppm, 3H, tripletarrow_forwardThe simulataed APT spectrum of a compound with the molecular formula C5H11Cl is shown. Draw a structure that is consistent with this data.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY