
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
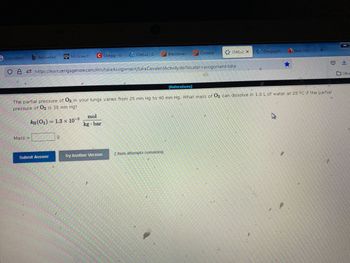
Transcribed Image Text:**Problem Description:**
The partial pressure of O₂ in your lungs varies from 25 mm Hg to 40 mm Hg. What mass of O₂ can dissolve in 1.0 L of water at 25 °C if the partial pressure of O₂ is 35 mm Hg?
**Given:**
- \( k_H(O₂) = 1.3 \times 10^{-3} \, \frac{\text{mol}}{\text{kg} \cdot \text{bar}} \)
**To Find:**
- Mass of \( O₂ \) in grams
**Instructions:**
Enter your answer in the box provided, and click "Submit Answer." You have 2 attempts remaining to answer the question correctly.
**Buttons:**
- Submit Answer
- Try Another Version
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give typed solution not writtenarrow_forwardg/mol PHY&CHEM HW Submit Answer Fall 2023 Tutoring S.. The freezing point of ethanol, CH₂CH₂OH, is -117.300 °C at 1 atmosphere. K(ethanol) = 1.99 °C/m In a laboratory experiment, students synthesized a new compound and found that when 13.05 grams of the compound were dissolved in 208.0 grams of ethanol, the solution began to freeze at -117.982 °C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? eview Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining Previous Nextarrow_forwardPls help ASAP. Pls circle the final answer.arrow_forward
- HQ12.22 X Answered. 1 attempt left Find the volume of HCI gas at STP required to prepare 3.50 L of a 0.100 m HCI solution (assume water density = 1.00 g/mL). Numeric answer 8.68 X X Answered - Incorrect Donearrow_forwardPbF2 3.3×10-8arrow_forwardest Ort a gamb rement: 56°F Cloudy Sodium chloride (NaCl) has a solubility of 36.0 g/100 g H₂O at 20 °C. Select the temperature at which the solubility of NaCl will be higher than 36.0 g/100 g H₂O among the following. U 20 °C O 10 °C O 40 °C chemistry and reaction of Employ... paymen... O 5 °C receipt % 10 f6 & hparrow_forward
- Helparrow_forwardCalculating molality A student dissolves 13. g of ammonia (NH3) in 175. mL of a solvent with a density of 0.98 g/mL. The student notices that the volume of the solvent does not change when the ammonia dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits. molarity = 0 = 0 molality Explanation Check x10 믐 X 8 0x0 S 0/5 MacBook Pro Val 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardChem 2 Hw : Show workarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY