
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Question 17
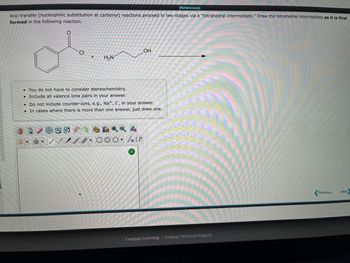
Transcribed Image Text:**Title: Understanding Tetrahedral Intermediates in Acyl Transfer Reactions**
In acyl transfer reactions (nucleophilic substitution at carbonyl), the reaction proceeds in two stages via a "tetrahedral intermediate." Here, we'll explore the formation of this intermediate through a given reaction.
**Reaction Overview:**
The reaction involves a compound with the structure:
- A benzene ring attached to a carbonyl group (C=O) with a chlorine (Cl) substituent on the carbonyl carbon.
- This is reacted with a molecule containing an amine group (NH2) and a hydroxyl group (OH).
**Steps to Draw the Tetrahedral Intermediate:**
1. **Identify the Reactants:**
- Reactant 1: A benzene ring attached to a carbonyl group (C=O) with a chlorine (Cl) substituent on the carbonyl carbon.
- Reactant 2: Molecule with an amine group (NH2) and a hydroxyl group (OH).
2. **Formation of the Intermediate:**
- The nucleophilic attack takes place when the amine nitrogen (NH2) attacks the carbonyl carbon (C=O) of the benzoyl chloride.
3. **After the Nucleophilic Attack:**
- This attack results in the addition of the nucleophile (NH2 group) to the carbonyl carbon, breaking the double bond (C=O) and forming a single bond (C-OH). The carbon now has four single bonds, making it a tetrahedral intermediate.
**Key Points:**
- You do not have to consider stereochemistry.
- Include all valence lone pairs in your drawing.
- Do not include counter-ions like \( \text{Na}^+ \) or \( \text{I}^- \).
- If more than one intermediate can be formed, just draw one.
**Diagrams:**
The diagram editor tools available will allow you to draw the various bonds and lone pairs needed to represent the tetrahedral intermediate accurately.
- **Benzene ring structure**: Use the hexagon symbol.
- **Carbonyl group (C=O)**: Draw the double bond from the hexagon (benzene) to the oxygen atom.
- **Chlorine substituent**: Draw the Cl atom on the carbonyl carbon before reaction and indicate its replacement after the nucleophilic attack.
- **
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I am required to enter answers in each box provided for the question. The answer that was provided is not correct.arrow_forwardPlease help me complete question 4,5,and6.arrow_forwardcorrect statement is? a. Safety glasses should be worn in a laboratory at all times to decrease the likelihood of eye injury. b. Safety shower would immediately be used if your clothing caught fire or if a large chemical spill had occurred on your clothing. O c. It is not allowed to smoke in the lab. o d. If you have accidentally broken a test tube and spilled a chemical on the table, Caution your lab partners to avoid the area while you inform the teacher of the small accident. e. Use the eye-wash fountain ,then notify the instructor if a chemical gets in the eye.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY